
Escitalopram Actavis

Ask a doctor about a prescription for Escitalopram Actavis

How to use Escitalopram Actavis
Leaflet accompanying the packaging: information for the user
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Escitalopram Actavis, 20 mg, film-coated tablets
Escitalopram
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including those not listed in the leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Escitalopram Actavis and what is it used for
- 2. Important information before taking Escitalopram Actavis
- 3. How to take Escitalopram Actavis
- 4. Possible side effects
- 5. How to store Escitalopram Actavis
- 6. Contents of the packaging and other information
1. What is Escitalopram Actavis and what is it used for
Escitalopram Actavis contains the active substance escitalopram. Escitalopram Actavis belongs to a group of antidepressants called selective serotonin reuptake inhibitors (SSRI). These medicines work on the serotonin system in the brain by increasing the level of serotonin. Disturbances in the serotonin system in the brain are thought to be involved in the development of depression and other mood disorders.
Escitalopram Actavis is used to treat depression (major depressive episodes), anxiety disorders (such as panic disorder with or without agoraphobia, social anxiety disorder, generalized anxiety disorder, and obsessive-compulsive disorder). It may take a few weeks before you start to feel better. You should continue to take Escitalopram Actavis even if it takes some time before you feel any improvement. If you do not feel better or feel worse, you should see your doctor.
2. Important information before taking Escitalopram Actavis
When not to take Escitalopram Actavis
Warnings and precautions
Before starting to take Escitalopram Actavis, you should tell your doctor or pharmacist. You should tell your doctor if you have other conditions or diseases, as your doctor should take this information into account. In particular, you should inform your doctor:
Warning
In some patients with bipolar affective disorder, a manic phase may occur. This is characterized by unusual and rapidly changing ideas, unjustified feelings of happiness, and excessive physical activity. If these symptoms occur, you should see your doctor.
In the first few weeks of treatment, symptoms such as restlessness or difficulty sitting or standing still may also occur. If such symptoms appear, you should immediately inform your doctor.
Medicines such as Escitalopram Actavis (so-called SSRI or SNRI) may cause symptoms of sexual dysfunction (see section 4). In some cases, these symptoms have persisted after discontinuation of treatment.
Suicidal thoughts and worsening of depression or anxiety disorder
In the case of depression and/or anxiety disorders, suicidal thoughts or self-harm thoughts may also occur. Such symptoms or behavior may worsen at the start of treatment with antidepressant medicines, as these medicines usually start to work only after about 2 weeks, sometimes later.
The likelihood of such thoughts occurring is higher if:
- the patient has had suicidal thoughts or self-harm thoughts before;
- the patient is an adult in young age. Information from clinical trials has shown an increased risk of suicidal behavior in adults under 25 years of age with mental disorders who were treated with antidepressant medicines.
If suicidal thoughts or self-harm thoughts ever occur, you should contact your doctor or go to the hospital immediately.
It may be helpful to inform your family or friendsabout your depression or anxiety disorder and ask them to read this leaflet. You may ask them to tell you if they notice that your depression or anxiety is getting worse or if you are experiencing worrying changes in your behavior.
Children and adolescents
Escitalopram Actavis should not be used in children and adolescents under 18 years of age.
It should also be noted that in the case of taking medicines from this group, patients under 18 years of age are at increased risk of side effects, such as suicidal attempts, suicidal thoughts, and hostility (especially aggression, rebellious behavior, and manifestations of anger). Nevertheless, the doctor may prescribe Escitalopram Actavis to patients under 18 years of age, considering that it is in their best interest. If a patient under 18 years of age has been prescribed Escitalopram Actavis and you have any doubts, you should contact your doctor again. If a patient under 18 years of age taking Escitalopram Actavis experiences or worsens any of the above symptoms, you should inform your doctor.
Additionally, the long-term safety of Escitalopram Actavis on growth, maturation, and cognitive development in this age group has not been established yet.
Escitalopram Actavis and other medicines
You should tell your doctor or pharmacist about all the medicines you are taking now or have taken recently and about the medicines you plan to take.
You should inform your doctor if you are taking any of the following medicines:
- non-selective monoamine oxidase inhibitors (MAOIs) containing substances such as phenelzine, iproniazid, isocarboxazid, nialamide, and tranylcypromine. If you have taken any of these medicines, you should wait 14 days before starting to take Escitalopram Actavis. After stopping treatment with Escitalopram Actavis, you should wait 7 days before taking any of these medicines;
- reversible, selective monoamine oxidase A inhibitors (MAO-A) containing moclobemide (a medicine used to treat depression);
- irreversible monoamine oxidase B inhibitors (MAO-B) containing selegiline (used to treat Parkinson's disease). These medicines increase the risk of side effects;
- the antibiotic linezolid;
- lithium (used to treat bipolar affective disorder) and tryptophan;
- imipramine and desipramine (used to treat depression);
- sumatriptan and similar medicines (used to treat migraine), tramadol, and buprenorphine (used to treat severe pain). These medicines may increase the risk of side effects;
- cimetidine, lansoprazole, omeprazole, and esomeprazole (used to treat stomach ulcers), fluconazole (a medicine used to treat fungal infections), fluvoxamine (an antidepressant), and ticlopidine (used to reduce the risk of stroke). These medicines may cause an increase in the level of escitalopram in the blood;
- St. John's wort (Hypericum perforatum) - a herbal medicine used to treat depression
- acetylsalicylic acid and non-steroidal anti-inflammatory medicines (medicines used to relieve pain or to thin the blood, known as anticoagulants). They may increase the tendency to bleed;
- warfarin, dipyridamole, and phenprocoumon (medicines used to thin the blood, known as anticoagulants). Your doctor may order a blood test to check the blood clotting time at the start and after stopping treatment with Escitalopram Actavis to determine if the dose of the anticoagulant is still appropriate;
- mefloquine (used to treat malaria), bupropion (used to treat depression), and tramadol (used to treat severe pain) due to the possible risk of lowering the seizure threshold;
- neuroleptics (medicines used to treat schizophrenia, psychosis) and antidepressant medicines (tricyclic antidepressants and SSRIs) due to the possible risk of lowering the seizure threshold;
- flecainide, propafenone, and metoprolol (used to treat heart conditions), desipramine, clomipramine, and nortriptyline (antidepressant medicines), and risperidone, thioridazine, and haloperidol (antipsychotic medicines). A dose adjustment of Escitalopram Actavis may be required;
- medicines that lower the level of potassium or magnesium in the blood, as they increase the risk of life-threatening heart rhythm disorders.
DO NOT TAKE Escitalopram Actavisif you are taking medicines used to treat heart conditions or medicines that may affect the heart rhythm, such as:
antiarrhythmic medicines of class IA and III, antipsychotic medicines (e.g., phenothiazine derivatives, pimozide, haloperidol), tricyclic antidepressant medicines, and some antibacterial medicines (e.g., sparfloxacin, moxifloxacin, intravenous erythromycin, pentamidine, antimalarial medicines, especially halofantrine), some antihistamine medicines (astemizole, hydroxyzine, mizolastine). If you have any doubts about taking the medicine, you should contact your doctor.
Escitalopram Actavis with food, drink, or alcohol
Escitalopram Actavis can be taken with or without food (see section 3 "How to take Escitalopram Actavis").
As with many medicines, it is not recommended to take Escitalopram Actavis and alcohol at the same time, although interactions between Escitalopram Actavis and alcohol are not expected.
Pregnancy, breastfeeding, and fertility
If you are pregnant, breastfeeding, or think you may be pregnant, or if you are planning to become pregnant, you should consult your doctor or pharmacist before taking this medicine.
You should not take Escitalopram Actavis during pregnancy and breastfeeding, unless you have discussed the risks and benefits of treatment with your doctor.
If you take Escitalopram Actavis during the last 3 months of pregnancy, you should be aware that the following symptoms may occur in the newborn: difficulty breathing, blue skin, seizures, changes in body temperature, difficulty feeding, vomiting, low blood sugar, muscle stiffness or floppiness, increased reflexes, tremors, nervousness, irritability, lethargy, crying, sleepiness, and difficulty sleeping. If any of these symptoms occur in the newborn, you should contact your doctor immediately.
You should inform your doctor and/or midwife about taking Escitalopram Actavis. Taking medicines like Escitalopram Actavis, especially in the last 3 months of pregnancy, may increase the risk of serious complications in the newborn called persistent pulmonary hypertension of the newborn (PPHN). This condition is characterized by rapid breathing and blue skin and usually occurs in the first day after birth. If such symptoms occur in the newborn, you should contact your doctor and/or midwife immediately.
You should not stop taking Escitalopram Actavis during pregnancy.
Taking Escitalopram Actavis at the end of pregnancy may increase the risk of severe bleeding from the vagina, which occurs shortly after delivery, especially if there is a history of bleeding disorders in the patient. If you take Escitalopram Actavis, you should inform your doctor or midwife so that they can give you appropriate advice.
It is assumed that escitalopram passes into breast milk.
In animal studies, it has been shown that citalopram, a medicine like escitalopram, affects sperm quality. This may theoretically affect fertility, but no effect on fertility has been observed in humans yet.
Driving and using machines
You should not drive or operate machinery until you know how Escitalopram Actavis affects you.
Escitalopram Actavis contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means that it is essentially "sodium-free".
3. How to take Escitalopram Actavis
This medicine should always be taken exactly as your doctor has told you. If you are not sure, you should ask your doctor or pharmacist.
Escitalopram Actavis is available in the following strengths: 10 mg, 15 mg, 20 mg.
Adults
Depression
The recommended dose of Escitalopram Actavis is 10 mg and is taken as one dose per day. The dose may be increased by your doctor to a maximum of 20 mg per day.
Panic disorder with agoraphobia
The initial dose of Escitalopram Actavis is 5 mg per day for the first week of treatment, then the dose is increased to 10 mg per day. The dose may then be increased by your doctor to a maximum of 20 mg per day.
Social anxiety disorder
The recommended dose of Escitalopram Actavis is 10 mg and is taken as one dose per day. The dose may then be decreased by your doctor to 5 mg per day or increased to a maximum of 20 mg per day, depending on the patient's response to the medicine.
Generalized anxiety disorder
The recommended dose of Escitalopram Actavis is 10 mg and is taken as one dose per day. The dose may be increased by your doctor to a maximum of 20 mg per day.
Obsessive-compulsive disorder
The recommended dose of Escitalopram Actavis is 10 mg and is taken as one dose per day. The dose may be increased by your doctor to a maximum of 20 mg per day.
Elderly patients (over 65 years of age)
The recommended initial dose of Escitalopram Actavis is 5 mg and is taken as one dose per day. The dose may be increased by your doctor to 10 mg per day.
Use in children and adolescents
Escitalopram Actavis should not normally be used in children and adolescents. For further information, see section 2 "Important information before taking Escitalopram Actavis".
Renal impairment
Care should be taken in patients with severe renal impairment. The medicine should be used as directed by your doctor.
Hepatic impairment
Patients with hepatic impairment should not exceed a dose of 10 mg per day. The medicine should be used as directed by your doctor.
Poor metabolizers of medicines that are metabolized by the CYP2C19 enzyme
Patients with this known genotype should not exceed a dose of 10 mg per day. The medicine should be used as directed by your doctor.
The film-coated tablets should be taken once a day, swallowed whole with sufficient fluid (preferably a glass of water). Escitalopram Actavis can be taken with or without food.
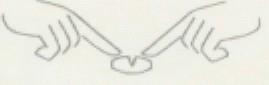
If necessary, the tablet can be divided into two equal doses by placing it on a flat surface with the score line facing upwards. The tablet can then be broken into two equal doses by pressing each end downwards with your index fingers, as shown in the picture.
Duration of treatment
You may not feel better until after a few weeks of treatment. Therefore, you should continue to take Escitalopram Actavis even if it takes some time before you feel any improvement. Do not change the dose without consulting your doctor.
Escitalopram Actavis should be taken for as long as your doctor has prescribed it. If you stop treatment too early, your symptoms may return. It is recommended to continue treatment for at least 6 months after you have felt better.
Taking a higher dose of Escitalopram Actavis than recommended
If you have taken more Escitalopram Actavis than prescribed, you should contact your doctor or go to the emergency department of the nearest hospital immediately. You should do this even if you do not feel any discomfort.
Symptoms of overdose include dizziness, tremors, agitation, coma, nausea, vomiting, heart rhythm disturbances, low blood pressure, and disturbances in water and electrolyte balance. When visiting your doctor or going to the hospital, you should take the packaging of Escitalopram Actavis with you.
Missing a dose of Escitalopram Actavis
You should not take a double dose to make up for a missed dose. If you have missed a dose and remember before going to bed, you should take the missed dose immediately. You should take the next dose at the usual time the next day. If you remember that you have missed a dose in the night or the next day, you should skip the missed dose and take the next dose as usual.
Stopping treatment with Escitalopram Actavis
You should not stop taking Escitalopram Actavis unless your doctor tells you to. When you stop treatment, it is usually recommended to gradually reduce the dose of Escitalopram Actavis over a few weeks.
After stopping treatment with Escitalopram Actavis, especially if it is stopped abruptly, you may experience withdrawal symptoms. These symptoms are common after stopping treatment with Escitalopram Actavis. The risk is higher if Escitalopram Actavis has been taken for a long time, in high doses, or if the dose has been reduced too quickly. In most patients, the symptoms are mild and disappear on their own within two weeks. In some patients, however, they may be more severe or longer-lasting (more than 2-3 months). If you experience severe withdrawal symptoms, you should contact your doctor. Your doctor may recommend restarting treatment with Escitalopram Actavis and reducing the dose more slowly.
Withdrawal symptoms include dizziness (unsteady gait, balance disturbances), tingling sensation, burning sensation, and (less commonly) electric shock sensations, also in the head, sleep disturbances (intensive dreams, nightmares, insomnia), anxiety, headache, nausea and/or vomiting, sweating (including night sweats), restlessness or agitation, tremors, disorientation, emotional instability or irritability, diarrhea, vision disturbances, palpitations (heart palpitations).
If you have any further doubts about taking the medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Escitalopram Actavis can cause side effects, although not everybody gets them.
Side effects usually disappear after a few weeks of treatment. You should remember that some of these side effects may also be symptoms of your illness and will disappear as you get better.
If you experience any of the following side effects during treatment, you should contact your doctor or go to the hospital immediately:
Uncommon(may affect up to 1 in 100 people):
- unusual bleeding, including gastrointestinal bleeding.
Rare(may affect up to 1 in 1,000 people):
- swelling of the skin, tongue, lips, throat, or face, hives, or difficulty breathing or swallowing (severe allergic reaction)
- high fever, agitation, confusion (disorientation), tremors, and sudden muscle contractions, which may be symptoms of a rare disorder called serotonin syndrome.
Frequency not known(frequency cannot be estimated from the available data)
- difficulty urinating
- seizures, see also section "Warnings and precautions"
- yellowing of the skin and whites of the eyes, which is a sign of liver damage or hepatitis
- rapid or irregular heartbeat, fainting, which may be symptoms of a life-threatening condition called Torsades de Pointes
- suicidal thoughts and behaviors, see also section "Warnings and precautions"
- sudden swelling of the skin or mucous membranes (angioedema)
The following side effects have also been reported:
Very common(may affect more than 1 in 10 people):
- nausea
- headache
Common(may affect up to 1 in 10 people):
- nasal congestion or sinusitis (sinus infection)
- decreased or increased appetite
- anxiety, restlessness, unusual dreams, difficulty sleeping, sleepiness, dizziness, yawning, tremors, tingling sensation
- diarrhea, constipation, vomiting, dry mouth
- increased sweating
- muscle and joint pain
- sexual dysfunction (delayed ejaculation, erectile dysfunction, decreased libido, and difficulty achieving orgasm in women)
- fatigue, fever
- weight gain
Uncommon(may affect up to 1 in 100 people):
- hives, rash, itching
- teeth grinding, agitation, nervousness, panic attacks, confusion (disorientation)
- sleep disturbances, taste disturbances, fainting (syncope)
- dilated pupils, vision disturbances, ringing in the ears (tinnitus)
- hair loss
- heavy menstrual bleeding
- irregular menstrual periods
- weight loss
- rapid heartbeat
- swelling of the arms or legs
- nosebleeds
Rare(may affect up to 1 in 1,000 people):
- aggression, depersonalization (feeling of loss of one's own identity, feeling of not being oneself), hallucinations
- slow heartbeat
Frequency not known(frequency cannot be estimated from the available data)
- decreased sodium levels in the blood (symptoms include nausea and malaise with muscle weakness or confusion)
- dizziness when standing up due to low blood pressure (orthostatic hypotension)
- abnormal liver function test results (increased liver enzyme activity in the blood)
- movement disorders (involuntary muscle movements)
- painful erection of the penis (priapism)
- symptoms of unusual bleeding in the skin and mucous membranes (ecchymoses)
- low platelet count (thrombocytopenia)
- increased secretion of antidiuretic hormone (ADH), leading to water retention in the body, dilutional hyponatremia, and decreased sodium levels
- galactorrhea (milk secretion) in men and women who are not breastfeeding
- severe bleeding from the vagina, which occurs shortly after delivery (postpartum hemorrhage), see additional information in subsection "Pregnancy, breastfeeding, and fertility" in section 2
- mania
- patients taking medicines from this group have been observed to have an increased risk of fractures
- changes in heart rhythm (called "QT interval prolongation", visible on an ECG, a test that evaluates heart function)
The following side effects are also known for medicines with a similar mechanism of action to escitalopram (the active substance of Escitalopram Actavis):
- restlessness (akathisia)
- loss of appetite
Reporting side effects
If you experience any side effects, including those not listed in the leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Escitalopram Actavis
The medicine should be kept out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Escitalopram Actavis contains
The active substance of Escitalopram Actavis is escitalopram. Each film-coated tablet contains 20 mg of escitalopram (as escitalopram oxalate).
The other ingredients of the medicine are:
Tablet core: microcrystalline cellulose, anhydrous colloidal silica, sodium croscarmellose, talc, magnesium stearate;
Tablet coating: hypromellose 6cP, titanium dioxide (E 171), macrogol 6000.
What Escitalopram Actavis looks like and contents of the pack
Oval, biconvex, white film-coated tablets (8 mm x 11.7 mm) with a score line on one side and notches on the sides, marked with "E" on the other side. The tablet can be divided into equal doses.
Escitalopram Actavis is available in blister packs containing 28, 30, 56, and 60 film-coated tablets in a cardboard box.
For more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Lithuania, the country of export:
Teva B.V., Swensweg 5, 2031 GA Haarlem, Netherlands
Manufacturer:
Actavis Ltd, BLB 015-016, Bulebel Industrial Estate, Zejtun ZTN 3000, Malta
Balkanpharma-Dupnitsa AD, 3 Samokovsko Shosse Str., Dupnitza 2600, Bulgaria
TjoaPack Netherlands B.V., Nieuwe Donk 9, 4879 AC Etten-Leur, Netherlands
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Marketing authorization number in Lithuania, the country of export:LT/1/09/1815/038
LT/1/09/1815/039
LT/1/09/1815/041
LT/1/09/1815/042
Parallel import authorization number: 180/24
Date of approval of the leaflet: 30.04.2024
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Teva B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Escitalopram ActavisDosage form: Tablets, 10 mgActive substance: escitalopramManufacturer: Biofarm Sp. z o.o.Prescription requiredDosage form: Tablets, 10 mgActive substance: escitalopramPrescription requiredDosage form: Tablets, 20 mgActive substance: escitalopramPrescription required
Alternatives to Escitalopram Actavis in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Escitalopram Actavis in Spain
Alternative to Escitalopram Actavis in Ukraine
Online doctors for Escitalopram Actavis
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Escitalopram Actavis – subject to medical assessment and local rules.











