

Ephedrinum hidrohloricum Vzf

Ask a doctor about a prescription for Ephedrinum hidrohloricum Vzf

How to use Ephedrinum hidrohloricum Vzf
Leaflet attached to the packaging: patient information
EPHEDRINUM HYDROCHLORICUM WZF, 25 mg/ml, solution for injection
Ephedrine hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Ephedrinum hydrochloricum WZF and what is it used for
- 2. Important information before using Ephedrinum hydrochloricum WZF
- 3. How to use Ephedrinum hydrochloricum WZF
- 4. Possible side effects
- 5. How to store Ephedrinum hydrochloricum WZF
- 6. Contents of the packaging and other information
1. What is Ephedrinum hydrochloricum WZF and what is it used for
Ephedrinum hydrochloricum WZF contains ephedrine, which dilates the smooth muscles of the bronchi and stimulates the respiratory center. Ephedrine accelerates the slowed heart rate and increases the strength of its contractions, as well as constricts peripheral blood vessels, which leads to an increase in blood pressure. Ephedrinum hydrochloricum WZF is used:
- in bronchial spasm conditions;
- in low blood pressure during spinal or epidural anesthesia.
2. Important information before using Ephedrinum hydrochloricum WZF
When not to use Ephedrinum hydrochloricum WZF:
Warnings and precautions
Before starting treatment with Ephedrinum hydrochloricum WZF, discuss it with your doctor or nurse. Ephedrine is administered only by medical personnel. The doctor will exercise particular caution when using ephedrine and take appropriate action:
If you experience shortness of breath and chest pain, you should contact your doctor immediately. You should inform your doctor before using this medicine if you have had heart disease, irregular heart rhythm, or chest pain in the past. After long-term administration of the medicine, there is no accumulation of the medicine in the body, but tolerance (which is a decrease in the effect of the medicine over time, requiring an increase in dose) and dependence (see section 4 "Possible side effects") have been observed. In patients with prostate enlargement, ephedrine may cause difficulty in urinating, up to urinary retention. Long-term use of ephedrine may cause anxiety. It is recommended to avoid long-term use of ephedrine in patients with neurosis. Ephedrine can be used in children with bronchial spasm, subcutaneously, and in the treatment of hypotension, intravenously after dilution. Ephedrine may give positive results in doping tests in athletes.
Ephedrinum hydrochloricum WZF and other medicines
Tell your doctor about all the medicines you are taking now or have taken recently, and about the medicines you plan to take, if possible and your condition allows. Do not use ephedrine with other medicines from the group of sympathomimetics (e.g., pseudoephedrine, phenylephrine, methylphenidate, phenylpropanolamine) due to the risk of acute hypertension. In patients receiving ephedrine and simultaneously taking cardiac glycosides, quinidine, or tricyclic antidepressants, the risk of arrhythmia increases. The effect of ephedrine is influenced by:
- antidepressant medicines called monoamine oxidase inhibitors (e.g., moclobemide, selegiline), especially if you have taken these medicines in the last 2 weeks;
- antidepressant medicines called selective serotonin and norepinephrine reuptake inhibitors (e.g., venlafaxine, duloxetine);
- tricyclic antidepressants (e.g., amitriptyline);
- linezolid (an antibacterial medicine);
- acetazolamide (used in the treatment of glaucoma) and other urine-alkalizing compounds;
- blood pressure-lowering medicines;
- doxapram (a medicine that stimulates, among other things, the respiratory system);
- guanidine and its derivatives (medicines used in diabetes, e.g., metformin);
- medicines from the group called cardiac glycosides, e.g., digoxin;
- medicines used for inhalation anesthesia, e.g., halothane, cyclopropane;
- oxytocin (a hormone administered during childbirth to accelerate it);
- medicines used in the treatment of asthma, e.g., salbutamol.
Ephedrine affects the action of:
- dexamethasone (an anti-inflammatory medicine);
- phenytoin (an antiepileptic medicine);
- barbiturates (sleeping medicines);
- primidone (an antiepileptic medicine).
Concomitant use with theophylline (a medicine that dilates the upper airways) may cause insomnia, excessive nervousness, and gastrointestinal discomfort.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before using this medicine. The use of the medicine during pregnancy will be decided by your doctor. You should not use Ephedrinum hydrochloricum WZF during breastfeeding.
Driving and using machines
Due to the condition that was an indication for the use of ephedrine (treatment of hypotension during anesthesia), the patient should not drive vehicles and operate machines. The medicine does not affect the ability to drive vehicles and operate machines. However, after doses much higher than recommended or in people who are particularly sensitive, ephedrine may cause excessive nervous excitement and have a negative impact on the performance of these activities.
3. How to use Ephedrinum hydrochloricum WZF
Ephedrinum hydrochloricum WZF is administered only by medical personnel.
- The dose of the medicine is determined by the doctor. The applied dose depends on the patient's age, body weight, and overall health.
- In bronchial spasm conditions in adults, ephedrine is administered intramuscularly or subcutaneously, in children only subcutaneously.
- In the treatment of hypotension, ephedrine is administered slowly intravenously after dilution.
- During intravenous administration of ephedrine, medical personnel monitor the patient's heart rate and blood pressure to check how the patient reacts to the medicine.
Using a higher dose of Ephedrinum hydrochloricum WZF than recommended
Ephedrine is administered by medical personnel, and therefore, it is unlikely that the patient will receive more medicine than they should. If the patient suspects that the dose of the medicine they received is too high, they should inform their doctor or nurse. After using a higher dose of ephedrine than recommended, the following may occur: nausea, vomiting, elevated body temperature, palpitations, tachycardia (accelerated heart rate), elevated blood pressure, hallucinations, delusions, and thinking disorders, decreased blood pressure, and anuria (anuria). Significant overdose can cause respiratory depression, convulsions, and coma. In case of these symptoms, the doctor will administer appropriate treatment.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Frequently (may occur less frequently than in 1 in 10 people): increased blood pressure, palpitations, accelerated heart rate, nervousness, irritability, anxiety, weakness, headaches, excessive sweating, difficulty falling asleep, confusion, anxiety, depression, shortness of breath, nausea, vomiting. Rarely (may occur less frequently than in 1 in 1,000 people): heart rhythm disorders, acute urinary retention. Frequency not known (cannot be estimated from the available data): angina pectoris (chest pain), slowed heart rate, cardiac arrest, decreased blood pressure, stroke, coagulation disorders, tremors, hypersensitivity, fear, paranoia, and hallucinations, episodes of closed-angle glaucoma, pulmonary edema, decreased appetite, diarrhea, abdominal pain, decreased potassium levels in the blood, changes in blood glucose levels. After long-term use, dependence may develop, which is associated with increased aggression and schizophrenia-like psychosis. After using doses of ephedrine higher than recommended, convulsions may occur. Additionally, difficulty in urinating, up to urinary retention, may occur, especially in patients with prostate enlargement, as well as rare cases of skin allergy in the form of rash.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of the medicine.
5. How to store Ephedrinum hydrochloricum WZF
Store the ampoules in the outer packaging to protect them from light, at a temperature below 25°C. Do not freeze. The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the carton and ampoule. The expiry date refers to the last day of the month stated. The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Ephedrinum hydrochloricum WZF contains
- The active substance of the medicine is ephedrine hydrochloride. Each 1 ml of the solution contains 25 mg of ephedrine hydrochloride.
- The other ingredients are: 10% hydrochloric acid (to adjust pH), water for injections.
What Ephedrinum hydrochloricum WZF looks like and what the packaging contains
Ephedrinum hydrochloricum WZF is a clear, colorless liquid. The packaging consists of a cardboard box containing 10 ampoules made of colorless glass with a capacity of 1 ml.
Marketing authorization holder and manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A., ul. Pelplińska 19, 83-200 Starogard Gdański, tel. +48 22 364 61 01. Date of the last update of the leaflet:December 2024.
Information intended only for healthcare professionals:
EPHEDRINUM HYDROCHLORICUM WZF, 25 mg/ml, solution for injection
Ephedrine hydrochloride
Preparation of Ephedrinum hydrochloricum WZF for administration and method of administration
- Ephedrine may be used intravenously only under medical supervision.
- Before intravenous administration, the medicinal product must be diluted.
- The medicine can be diluted in aseptic conditions with 0.9% NaCl solution. The sodium content from the diluent should be taken into account when calculating the sodium content in the prepared dilution of the medicine. To obtain accurate information about the sodium content in the solution used to dilute the medicine, you should read the patient information leaflet of the diluent used.
- To obtain a solution containing 5 mg of ephedrine in 1 ml, the contents of the ampoule (1 ml) should be diluted in 0.9% NaCl solution to a volume of 5 ml of the solution.
- To obtain a solution containing 2.5 mg of ephedrine in 1 ml, the contents of the ampoule (1 ml) should be diluted in 0.9% NaCl solution to a volume of 10 ml of the solution.
- For microbiological reasons, the solution for injection should be prepared immediately before administration.
- If necessary, on the user's responsibility, the prepared solution can be stored for a maximum of 24 hours at a temperature below 25°C, provided that the dilution is prepared in controlled and validated aseptic conditions.
- Unused solution within 24 hours should be discarded.
Precautions for the use of Ephedrinum hydrochloricum WZF
- Ephedrine should be used with caution in patients with angina pectoris due to its positive chronotropic and inotropic effects.
- Sympathomimetic medicines, including Ephedrinum hydrochloricum WZF, may affect the cardiovascular system. Data from post-marketing studies and published in the literature indicate rare cases of myocardial ischemia associated with the use of beta-agonist medicines. Patients with severe heart disease (e.g., arrhythmia, tachycardia, severe heart failure) who receive Ephedrinum hydrochloricum WZF should be informed to seek medical advice if they experience chest pain or other symptoms of worsening heart disease. Attention should be paid to symptoms such as shortness of breath and chest pain, as they may come from both the respiratory and cardiac systems. Particular attention should also be paid to the use of ephedrine in patients with atherosclerosis, hypertension, and aneurysm, as well as other conditions involving narrowing of the blood vessels.
- In patients receiving ephedrine and simultaneously taking cardiac glycosides, quinidine, or tricyclic antidepressants, the risk of arrhythmia increases.
- Cautious use is recommended in patients with diabetes, as ephedrine increases the risk of hypoglycemia.
- Cautious use is recommended in patients with closed-angle glaucoma.
- In patients with prostate enlargement, ephedrine may cause difficulty in urinating, up to urinary retention, due to the contraction of the sphincter and simultaneous relaxation of the urinary bladder.
- Long-term use of ephedrine may cause anxiety. It is recommended to avoid long-term use of ephedrine in patients with neurosis. Children are less susceptible to the stimulating effect of ephedrine.
- After long-term administration of the product, there is no accumulation of the product in the body, but tolerance and dependence have been observed.
- Ephedrine may give positive results in doping tests in athletes.
Instructions for opening the ampoule
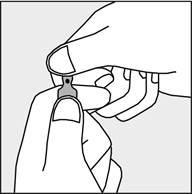
Before opening the ampoule, make sure that the entire solution is in the lower part of the ampoule. You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution. Each ampoule has a colored dot (see Figure 1) as a mark indicating the break point below it.
- To open the ampoule, hold it vertically, with both hands, with the colored dot facing you - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
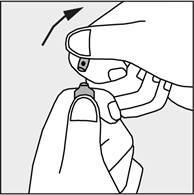
- Press in the direction of the arrow shown in Figure 3. The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused product should be destroyed in accordance with applicable regulations.
Figure 1
Figure 2
Figure 3
Dosage
Bronchial spasm conditions
Adults:
Intramuscularly or subcutaneously from 12.5 mg to 25 mg. Up to 150 mg in 24 hours in divided doses.
Children:
Subcutaneously 3 mg/kg body weight per day or 25 to 100 mg/m2 per day in 4-6 divided doses.
Hypotension during spinal or epidural anesthesia
Adults and children over 12 years old:
Intravenously, only after dilution (dilution method see above). Administer slowly, usually in a dose of 2.5 mg to 5 mg (up to 10 mg). Depending on the effect achieved, doses can be repeated every 3-4 minutes, up to a maximum dose of 30 mg. If the administration of 30 mg of ephedrine does not produce the expected effect, consider choosing another medicine. Do not exceed the maximum daily dose of 150 mg in divided doses.
Children under 12 years old:
Intravenously, only after dilution (dilution method see above). The medicine should be administered slowly in a dose of 0.5 to 0.75 mg/kg body weight or 17-25 mg/m2, if necessary, doses can be repeated every 3-4 minutes, depending on the effect achieved.
Elderly:
There is no need to modify the dosage. In justified cases, in adults, before performing central blockade, ephedrine may be administered prophylactically, intramuscularly without dilution, in a dose of 12.5 mg to 25 mg.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ephedrinum hidrohloricum Vzf
Alternatives to Ephedrinum hidrohloricum Vzf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ephedrinum hidrohloricum Vzf in Spain
Online doctors for Ephedrinum hidrohloricum Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ephedrinum hidrohloricum Vzf – subject to medical assessment and local rules.









