
Eltrombopag Vipharm

Ask a doctor about a prescription for Eltrombopag Vipharm

How to use Eltrombopag Vipharm
Leaflet accompanying the packaging: information for the user
Eltrombopag Vipharm, 12.5 mg, film-coated tablets
Eltrombopag Vipharm, 25 mg, film-coated tablets
Eltrombopag Vipharm, 50 mg, film-coated tablets
Eltrombopag Vipharm, 75 mg, film-coated tablets
Eltrombopag
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- The leaflet should be kept in case it needs to be read again.
- In case of any doubts, the doctor or pharmacist should be consulted.
- This medicine has been prescribed specifically for one person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Eltrombopag Vipharm and what is it used for
- 2. Important information before taking Eltrombopag Vipharm
- 3. How to take Eltrombopag Vipharm
- 4. Possible side effects
- 5. How to store Eltrombopag Vipharm
- 6. Contents of the pack and other information
1. What is Eltrombopag Vipharm and what is it used for
Eltrombopag Vipharm contains eltrombopag, which belongs to a group of medicines called thrombopoietin receptor agonists. The medicine is used to increase the number of platelets in the patient's blood.
- Eltrombopag Vipharm is used to treat a bleeding disorder called primary immune thrombocytopenia (ITP) in patients over 1 year of age who have been previously treated with other medicines (corticosteroids or immunoglobulins) and have not responded to them.
Primary immune thrombocytopenia is caused by a low platelet count (thrombocytopenia). People with primary immune thrombocytopenia are more prone to bleeding. Symptoms that patients with primary immune thrombocytopenia may notice include petechiae (small, flat, red spots under the skin), purpura, nosebleeds, gum bleeding, and difficulty stopping bleeding in case of injury or trauma.
- Eltrombopag Vipharm may also be used to treat low platelet count (thrombocytopenia) in adults with hepatitis C virus (HCV) infection who have had difficulty due to side effects during treatment with interferon. Many people with hepatitis C have a low platelet count, not only due to the disease but also as a result of the action of certain antiviral drugs used in treatment. Taking Eltrombopag Vipharm may help patients complete a full course of antiviral treatment (peginterferon and ribavirin).
- Eltrombopag Vipharm can also be used to treat adults with low platelet count caused by severe aplastic anemia (severe aplastic anemia, SAA). Severe aplastic anemia is a disease in which the bone marrow is damaged, leading to a deficiency of red blood cells (anemia), white blood cells (leukopenia), and platelets (thrombocytopenia).
...
2. Important information before taking Eltrombopag Vipharm
When not to take Eltrombopag Vipharm
- If the patient is allergicto eltrombopag or any of the other ingredients of this medicine (listed in section 6). Consult a doctorif the patient thinks they have this condition.
Warnings and precautions
Before starting treatment with Eltrombopag Vipharm, the doctor should be consulted:
- If the patient has liver disease. People with a low platelet count, as well as advanced (long-standing) liver disease, are at higher risk of side effects, including life-threatening liver damage and blood clots. If the doctor considers that the benefits of taking Eltrombopag Vipharm outweigh the risks, the patient will be closely monitored during treatment;
- If the patient has a risk of blood clots in the veins or arteries, or if there have been cases of blood clots in the family.
Risk of blood clots may be increased:
- If the patient is elderly
- If the patient has been immobile for a long time
- If the patient has cancer
- If the patient is taking oral contraceptives or hormone replacement therapy
- If the patient has recently undergone surgery or trauma
- If the patient is overweight
- If the patient smokes
- If the patient has advanced chronic liver diseaseTell your doctorbefore starting treatment if any of the above conditions apply to the patient. Do not take Eltrombopag Vipharm unless the doctor considers that the expected benefits outweigh the risk of blood clots.
- If the patient has cataracts
- If the patient has other blood disorders, such as myelodysplastic syndrome (MDS). Before starting treatment with Eltrombopag Vipharm, the doctor will perform tests to rule out this disease. If the patient has MDS and is taking Eltrombopag Vipharm, MDS may worsen. Tell your doctorif any of the above situations apply to the patient.
Ophthalmological examination
The doctor will recommend an eye examination to detect cataracts. If the patient does not undergo routine eye examinations, the doctor should recommend regular examinations. The occurrence of any bleeding in the retina (layer of light-sensitive cells at the back of the eye) or near it may also be examined.
Regular examinations will be necessary
Before starting treatment with Eltrombopag Vipharm, the doctor will perform blood tests to assess blood cells, including platelets. During treatment, these tests will be repeated at regular intervals.
Blood tests to assess liver function
Eltrombopag Vipharm may cause changes in blood test results that may indicate liver damage - increased activity of certain liver enzymes, in particular bilirubin and alanine and aspartate transaminases. If the patient is taking interferon-based treatment with Eltrombopag Vipharm for low platelet count associated with hepatitis C, some liver diseases may worsen.
Before starting treatment with Eltrombopag Vipharm and at regular intervals during treatment, the patient will undergo blood tests to assess liver function. It may be necessary to discontinue Eltrombopag Vipharm if the levels of these substances increase too much or if other symptoms of liver damage occur.
See the information in section 4 of this leaflet "Liver disorders".
Blood test to assess platelets
If the patient stops taking Eltrombopag Vipharm, there is a likelihood that the platelet count will decrease again within a few days. The platelet count will be monitored, and the doctor will discuss appropriate precautions with the patient.
A very high platelet count may increase the risk of blood clots. However, blood clots can also occur when the platelet count is normal or too low. The doctor will adjust the dose of Eltrombopag Vipharm for the patient to prevent excessive increase in platelet count.

Seek immediate medical attentionif the patient experiences any of the following symptoms of a blood clot:
- Swelling, painor tenderness of one leg
- Sudden shortness of breath, especially with severe chest pain or rapid breathing
- Abdominal pain (stomach), abdominal distension, blood in stool
Examinations to assess bone marrow
In people with bone marrow disorders, medicines like Eltrombopag Vipharm may worsen these disorders. Changes in bone marrow may be indicated by abnormal blood test results. The doctor may order direct bone marrow examinations during treatment with Eltrombopag Vipharm.
Examinations to detect gastrointestinal bleeding
If the patient is taking interferon-based treatment with Eltrombopag Vipharm, they will be monitored for symptoms of gastrointestinal bleeding after stopping Eltrombopag Vipharm.
Heart examinations
The doctor may consider the need for a heart examination during treatment with Eltrombopag Vipharm and perform an electrocardiogram (ECG).
Elderly patients (65 years and older)
There is limited data on the use of Eltrombopag in patients aged 65 and older. Caution should be exercised when taking Eltrombopag Vipharm in patients aged 65 and older.
Children and adolescents
Eltrombopag Vipharm is not recommended for children under 1 year of age with primary immune thrombocytopenia.
It is also not recommended for patients under 18 years of age with low platelet count associated with hepatitis C virus infection or severe aplastic anemia.
Eltrombopag Vipharm and other medicines
The doctor or pharmacist should be told about all medicines the patient is currently taking or has recently taken, as well as any medicines the patient plans to take. This includes medicines obtained without a prescription and vitamins.
Some commonly used medicines interact with Eltrombopag Vipharm- including both prescription and over-the-counter medicines and mineral supplements. These include:
- Antacids used to treat indigestion, heartburn, stomach ulcers
- Stomach(see also "When to take the medicine" in section 3)
- Medicines called statins, which lower cholesterol levels
- Certain medicines used to treat HIV infection, such as lopinavir and (or) ritonavir
- Cyclosporine used in transplantsor in immune system disorders
- Mineral products, such as iron, calcium, magnesium, aluminum, selenium, and zinc, which may be ingredients in vitamin and mineral supplements(see also "When to take the medicine" in section 3)
- Medicines such as methotrexate and topotecan, used to treat cancerTell your doctorif the patient is taking any of the above medicines. Some of them should not be taken during treatment with Eltrombopag Vipharm, while others may require a dose adjustment or appropriate timing of administration. The doctor will review the patient's medicines and recommend changes if necessary.
If the patient is taking medicines to prevent blood clots, there is an increased risk of bleeding. The doctor will discuss this with the patient.
If the patient is taking corticosteroids, danazol, and (or) azathioprine, the doses of these medicines may be reduced or their use may be discontinued during concurrent use of Eltrombopag Vipharm.
Taking Eltrombopag Vipharm with food and drink
Eltrombopag Vipharm should not be taken with dairy products or drinks, as the calcium in dairy products affects the absorption of the medicine. For more information, see "When to take the medicine" in section 3.
Pregnancy and breastfeeding
Do not take Eltrombopag Vipharm during pregnancy, unless the doctor recommends it. The effect of eltrombopag during pregnancy is unknown.
- Tell your doctorif the patient is pregnant, thinks they may be pregnant, or plans to have a baby.
- Use appropriate contraceptionwhile taking Eltrombopag Vipharm to prevent pregnancy.
- Tell your doctorif the patient becomes pregnant while taking Eltrombopag Vipharm.
Do not breastfeed while taking Eltrombopag Vipharm. It is not known whether eltrombopag passes into breast milk.
Tell your doctorif the patient is breastfeeding or plans to breastfeed.
Driving and using machines
Eltrombopag Vipharm may cause dizzinessand other side effects that reduce attention.
Do not drive or operate machineryunless the patient is sure that these symptoms do not occur.
Eltrombopag Vipharm contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per film-coated tablet, which means the medicine is essentially "sodium-free".
3. How to take Eltrombopag Vipharm
This medicine should always be taken as directed by the doctor. In case of doubts, the doctor or pharmacist should be consulted. The dose or dosing schedule of Eltrombopag Vipharm should not be changed unless the doctor or pharmacist recommends it. During treatment with Eltrombopag Vipharm, the patient will remain under the care of a doctor experienced in treating the disease.
How much medicine to take
Primary immune thrombocytopenia
Adults and children(aged 6 to 17 years) - the usual starting dose for primary immune thrombocytopenia is one 50 mg tabletof Eltrombopag Vipharm per day.
For patients of East Asian or Southeast Asian descent, it may be necessary to start treatment with a lower dose of 25 mg.
Children(aged 1 to 5 years) - the usual starting dose for primary immune thrombocytopenia is one 25 mg tabletof Eltrombopag Vipharm per day.
Other pharmaceutical forms may be more suitable for this patient group.
Hepatitis C
Adults- the usual starting dose for hepatitis C is one 25 mg tabletof Eltrombopag Vipharm per day. For patients of East Asian or Southeast Asian descent, treatment should be started with the same dose of 25 mg.
Severe aplastic anemia
Adults- the usual starting dose for severe aplastic anemia is one 50 mg tabletof Eltrombopag Vipharm per day. For patients of East Asian or Southeast Asian descent, it may be necessary to start treatment with a lower dose of 25 mg.
How to take the tablets
The tablets should be swallowed whole with water.
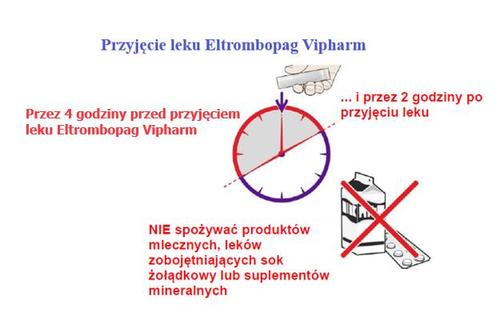
When to take the medicine
Make sure that:
→ 4 hours beforetaking Eltrombopag Vipharm
→ and 2 hours aftertaking Eltrombopag Vipharm
the patient does not consumethe following foods:
- Dairy products, such as cheese, butter, yogurt, ice cream
- Milkor drinks containing milk, yogurt, or cream
- Antacidsused to treat indigestion and heartburn
- Vitamin and mineral supplementscontaining iron, calcium, magnesium, aluminum, selenium, and zinc
If these recommendations are not followed, Eltrombopag Vipharm will not be properly absorbed by the body.
Talk to your doctor for more advice on suitable foods and drinks.
Taking more than the recommended dose of Eltrombopag Vipharm
If more than the recommended dose of Eltrombopag Vipharm is taken
Contact a doctor or pharmacist immediately. If possible, show the medicine packaging or leaflet. The patient's condition will be monitored for potential side effects and appropriate treatment will be administered promptly.
Missing a dose of Eltrombopag Vipharm
Take the next dose at the usual time. Do not take more than one dose of Eltrombopag Vipharm per day.
Stopping treatment with Eltrombopag Vipharm
Do not stop taking Eltrombopag Vipharm without consulting a doctor first. If the doctor recommends stopping treatment, the patient's platelet count will be monitored weekly for four weeks. See also "Bleeding or bruising after stopping treatment" in section 4.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Symptoms to watch out for: see a doctor
In patients taking Eltrombopag Vipharm for primary immune thrombocytopenia or low platelet count associated with hepatitis C, symptoms of severe side effects may occur. It is essential to inform the doctor about these symptoms.
Increased risk of blood clots
In some patients, there may be an increased risk of blood clots, and medicines like Eltrombopag Vipharm may worsen this condition. Sudden blockage of a blood vessel by a blood clot is an uncommon side effect and may occur in up to 1 in 100 patients.

Seek immediate medical attention if the patient experiences symptoms of a blood clot, such as:
- Swelling, pain, warmth, redness, or tenderness of one leg
- Sudden shortness of breath, especially with severe chest pain or rapid breathing
- Abdominal pain (stomach), abdominal distension, blood in stool
Liver disorders
Eltrombopag Vipharm may cause changes in blood test results that may indicate liver damage. Liver disorders (increased enzyme activity in blood test results) are common and may occur in up to 1 in 10 patients. Other liver problems are uncommon and may occur in up to 1 in 100 patients.
If any of the following symptoms of liver disorders occur:
- Yellowing of the skinor whites of the eyes (jaundice)
- Dark urine
Inform your doctor immediately.
Bleeding or bruising after stopping treatment
Usually, within two weeks of stopping Eltrombopag Vipharm, the patient's platelet count decreases to pre-treatment levels. A low platelet count may increase the risk of bleeding or bruising. The doctor will monitor the patient's platelet count for at least 4 weeks after stopping Eltrombopag Vipharm.
Tell your doctorif the patient experiences bruising or bleeding after stopping Eltrombopag Vipharm.
In some patients, gastrointestinal bleedingmay occur after stopping peginterferon, ribavirin, and eltrombopag. Symptoms include:
- Black, tarry stools (a change in stool color, which is an uncommon side effect that may occur in up to 1 in 100 patients)
- Blood in stool
- Vomiting blood or coffee-ground-like material
Tell your doctor immediatelyif any of these symptoms occur.
The following side effects have been reported in adults taking eltrombopag for primary immune thrombocytopenia:
Very common side effects
May occur in more than 1 in 10patients:
- Cold
- Nausea
- Diarrhea
- Cough
- Upper respiratory tract infection (infection of the nose, throat, and upper airways)
- Back pain
Very common side effects that may be seen in blood tests:
- Increased activity of liver enzymes (alanine transaminase (ALT))
Common side effects
May occur in up to 1 in 10patients:
- Muscle pain, muscle spasms, muscle weakness
- Bone pain
- Heavy menstrual bleeding
- Sore throat and discomfort when swallowing
- Eye disorders, including abnormal eye test results, dry eyes, eye pain, and blurred vision
- Vomiting
- Flu
- Cold sore
- Pneumonia
- Sinusitis (inflammation of the sinuses)
- Tonsillitis (inflammation of the tonsils)
- Respiratory tract infection (infection of the nose, throat, and airways)
- Gingivitis (inflammation of the gums)
- Loss of appetite
- Numbness, tingling, or prickling sensations
- Reduced skin sensation
- Drowsiness
- Ear pain
- Pain, swelling, or tenderness of one leg (usually the calf, with skin redness and warmth, symptoms of deep vein thrombosis)
- Localized swelling filled with blood from a damaged blood vessel (hematoma)
- Hot flashes
- Mouth disorders, including dry mouth, mouth pain, tongue sensitivity, gum bleeding, and mouth ulcers
- Runny nose
- Toothache
- Abdominal pain
- Abnormal liver function
- Skin changes, including excessive sweating, rash, redness, itching, and skin discoloration
- Hair loss
- Protein in the urine
- High fever
- Chest pain
- Weakness
- Difficulty sleeping, depression
- Migraine
- Blurred vision
- Dizziness
- Gas
Common side effects that may be seen in blood tests:
- Decreased red blood cell count (anemia)
- Decreased platelet count (thrombocytopenia)
- Decreased white blood cell count
- Decreased hemoglobin level
- Increased eosinophil count
- Increased white blood cell count (leukocytosis)
- Increased uric acid level
- Decreased potassium level
- Increased creatinine level
- Increased alkaline phosphatase activity
- Increased aspartate transaminase (AST) activity
- Increased bilirubin level in the blood (a substance produced by the liver)
- Increased levels of certain proteins
Uncommon side effects
May occur in up to 1 in 100patients:
- Allergic reaction
- Heart attack (interrupted blood flow to part of the heart)
- Sudden shortness of breath, especially with severe chest pain or rapid breathing, which may be a symptom of a blood clot in the lungs (see "Increased risk of blood clots" above in section 4)
- Lung tissue death due to a blockage of the blood supply (pulmonary embolism)
- Possible pain, swelling, or redness in the leg, which may be symptoms of a blood clot in a deep vein
- Yellowing of the skin or whites of the eyes (jaundice), abdominal pain, which may be symptoms of liver damage or blockage of the bile ducts
- Liver damage caused by the medicine
- Abnormal heart rhythm (prolonged QT interval), which may be a symptom of a heart disorder
- Blood clots
- Redness
- Pain
Uncommon side effects that may be seen in blood tests:
- Changes in red blood cell shape
- Presence of immature white blood cells, which may indicate certain diseases
- Increased platelet count
- Decreased calcium level
- Decreased red blood cell count (anemia) due to excessive destruction of red blood cells (hemolytic anemia)
- Increased myelocyte count
- Increased neutrophil count
- Increased urea level in the blood
- Increased protein level in the urine
- Increased albumin level in the blood
- Increased total protein level
- Decreased albumin level in the blood
- Increased urine pH
- Increased hemoglobin level
Side effects with unknown frequency
Frequency cannot be estimated from the available data
- Skin discoloration
- Darker skin color
- Liver damage caused by the medicine
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the doctor, pharmacist, or nurse should be informed. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
PL-02 222 Warsaw
Tel: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Eltrombopag Vipharm
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and blister. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
Eltrombopag Vipharm 12.5 mg and 25 mg film-coated tablets do not require any special storage conditions.
Eltrombopag Vipharm 50 mg and 75 mg film-coated tablets do not require any special storage conditions; store in the original packaging to protect from light.
6. Contents of the pack and other information
What Eltrombopag Vipharm contains
The active substance of Eltrombopag Vipharm is eltrombopag.
12.5 mg film-coated tablets
Each film-coated tablet contains eltrombopag olamine equivalent to 12.5 mg of eltrombopag.
25 mg coated tablets
Each coated tablet contains eltrombopag olamine equivalent to 25 mg of eltrombopag.
50 mg coated tablets
Each coated tablet contains eltrombopag olamine equivalent to 50 mg of eltrombopag.
75 mg coated tablets
Each coated tablet contains eltrombopag olamine equivalent to 75 mg of eltrombopag.
The other ingredients are: mannitol (E421), microcrystalline cellulose, povidone, sodium carboxymethyl starch, magnesium stearate, titanium dioxide (E171), hypromellose (E464), macrogol (E1521).
Eltrombopag Vipharm 12.5 mg and 25 mg coated tablets also contain polysorbate 80 (E433).
Eltrombopag Vipharm 50 mg coated tablets also contain red iron oxide (E 172) and yellow iron oxide (E 172).
Eltrombopag Vipharm 75 mg coated tablets also contain red iron oxide (E 172) and black iron oxide (E 172).
What Eltrombopag Vipharm looks like and what the pack contains
Eltrombopag Vipharm 12.5 mg coated tablets, white, round, biconvex coated tablets with the inscription "12.5" on one side and smooth on the other side.
Eltrombopag Vipharm 25 mg coated tablets, white, round, biconvex with the inscription "25" on one side and smooth on the other side.
Eltrombopag Vipharm 50 mg coated tablets, brown, round, biconvex with the inscription "50" on one side and smooth on the other side.
Eltrombopag Vipharm 75 mg coated tablets, pink, round, biconvex with the inscription "75" on one side and smooth on the other side.
The tablets are supplied in aluminum blisters packaged in a cardboard box containing 14, 28 or 48 coated tablets.
Not all pack sizes may be available in your country.
Marketing Authorisation Holder
Vipharm S.A.
ul. A. i F. Radziwiłłów 9
05-850 Ożarów Mazowiecki
Poland
e-mail: [email protected]
Manufacturer
Genepharm S.A.
18th km Marathonos Avenue,
153 51 Pallini
Greece
This medicinal product is authorised in the Member States of the European Economic Area under the following names:
Economic Area under the following names:
Denmark:
Eltrombopag Vipharm
Czech Republic:
Eltrombopag Vipharm
Hungary:
Eltrombopag Vipharm 12.5 mg, 25 mg, 50 mg, 75 mg film-coated tablets
Poland:
Eltrombopag Vipharm
Slovakia:
Eltrombopag Vipharm 12.5 mg, 25 mg, 50 mg, 75 mg film-coated tablets
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGenepharm S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Eltrombopag VipharmDosage form: Tablets, 25 mgActive substance: eltrombopagPrescription requiredDosage form: Tablets, 50 mgActive substance: eltrombopagPrescription requiredDosage form: Tablets, 25 mgActive substance: eltrombopagPrescription required
Alternatives to Eltrombopag Vipharm in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Eltrombopag Vipharm in Spain
Alternative to Eltrombopag Vipharm in Ukraine
Online doctors for Eltrombopag Vipharm
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Eltrombopag Vipharm – subject to medical assessment and local rules.














