
Eltrombopag Polpharma

Ask a doctor about a prescription for Eltrombopag Polpharma

How to use Eltrombopag Polpharma
Leaflet accompanying the packaging: information for the user
Eltrombopag Polpharma, 12.5 mg, film-coated tablets
Eltrombopag Polpharma, 25 mg, film-coated tablets
Eltrombopag Polpharma, 50 mg, film-coated tablets
Eltrombopag Polpharma, 75 mg, film-coated tablets
Eltrombopag
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Eltrombopag Polpharma and what is it used for
- 2. Important information before taking Eltrombopag Polpharma
- 3. How to take Eltrombopag Polpharma
- 4. Possible side effects
- 5. How to store Eltrombopag Polpharma
- 6. Contents of the packaging and other information
1. What is Eltrombopag Polpharma and what is it used for
Eltrombopag Polpharma contains eltrombopag, which belongs to a group of medicines called thrombopoietin receptor agonists. The medicine is used to increase the number of platelets in the patient's blood. Platelets are blood components that help reduce the risk of bleeding or prevent it. Eltrombopag Polpharma is used to treat a blood clotting disorder called primary immune thrombocytopenia (ITP) in patients over 1 year of age who have already been treated with other medicines (corticosteroids or immunoglobulins) and have not responded to them. Primary immune thrombocytopenia is caused by a low number of platelets in the blood (thrombocytopenia). People with primary immune thrombocytopenia are more prone to bleeding. Symptoms that patients with primary immune thrombocytopenia may notice include petechiae (small, flat, red spots under the skin), subcutaneous hemorrhages, nosebleeds, gum bleeding, and inability to stop bleeding in case of injury or trauma. Eltrombopag Polpharma may also be used to treat low platelet count (thrombocytopenia) in adults with hepatitis C virus (HCV) infection who have had difficulties due to side effects during interferon treatment. Many people with hepatitis C have a low platelet count, not only due to the disease but also as a result of the action of some antiviral drugs used in its treatment. Taking Eltrombopag Polpharma may help patients complete full antiviral treatment (peginterferon and ribavirin).
2. Important information before taking Eltrombopag Polpharma
When not to take Eltrombopag Polpharma
- -if the patient is allergic to eltrombopag or any of the other ingredients of this medicine (listed in section 6 under "What Eltrombopag Polpharma contains"). The patient should consult their doctor if they think they have this condition.
Warnings and precautions
Before starting treatment with Eltrombopag Polpharma, the patient should discuss the following with their doctor:
- if the patient has liver disease. People with a low platelet count and advanced (long-term) liver disease are at higher risk of side effects, including life-threatening liver damage and blood clots. If the doctor considers that the benefits of taking Eltrombopag Polpharma outweigh the risks, the patient will be closely monitored during treatment.
- if the patient has a risk of blood clots in the veins or arteries, or if there have been cases of blood clots in their family.
The risk of blood clots may be increased:
- if the patient is elderly
- if the patient has been immobilized for a long time
- if the patient has cancer
- if the patient is taking oral contraceptives or hormone replacement therapy
- if the patient has recently undergone surgery or had an injury
- if the patient is overweight
- if the patient smokes
- if the patient has advanced chronic liver disease. The patient should inform their doctor before starting treatmentif any of the above conditions apply to them. The patient should not take Eltrombopag Polpharma unless their doctor considers that the expected benefits outweigh the risk of blood clots.
- if the patient has cataracts(clouding of the lens in the eye)
- if the patient has other blood disorders, such as myelodysplastic syndrome (MDS). Before starting treatment with Eltrombopag Polpharma, the doctor will perform tests to rule out this condition. If the patient has MDS and takes Eltrombopag Polpharma, MDS may worsen.
The patient should inform their doctor if any of the above situations apply to them.
Eye examination
The doctor will recommend an eye examination to detect cataracts. If the patient does not undergo regular eye examinations, the doctor should recommend regular examinations. The occurrence of any bleeding in the retina (the light-sensitive layer at the back of the eye) or near it may also be examined.
Regular tests will be necessary
Before starting treatment with Eltrombopag Polpharma, the doctor will perform blood tests to assess the patient's blood cells, including platelets. During treatment, these tests will be repeated at regular intervals.
Blood tests for liver function
Eltrombopag Polpharma may cause changes in blood test results that may indicate liver damage - increased activity of certain liver enzymes, particularly bilirubin and alanine and aspartate transaminases. If the patient is taking interferon-based treatment and Eltrombopag Polpharma for low platelet count associated with hepatitis C, some liver diseases may worsen. Blood tests to assess liver function will be performed before starting treatment with Eltrombopag Polpharma and at regular intervals during treatment. It may be necessary to stop taking Eltrombopag Polpharma if the levels of these substances increase too much or if other symptoms of liver damage occur. The patient should read the information in section 4 of this leaflet "Liver disorders".
Platelet count tests
If the patient stops taking Eltrombopag Polpharma, it is likely that the low platelet count will return within a few days. A low platelet count may increase the risk of bleeding or bruising. The doctor will monitor the patient's platelet count and discuss appropriate precautions.
Blood clot tests
A very high platelet count may increase the risk of blood clots. However, blood clots can also occur when the platelet count is normal or too low. The doctor will adjust the dose of Eltrombopag Polpharma to prevent the platelet count from becoming too high. The patient should seek immediate medical attentionif they experience any of the following symptoms of a blood clot:
- swelling, pain, or tenderness in one leg
- sudden shortness of breath, especially with sudden chest pain or rapid breathing
- abdominal pain, abdominal enlargement, blood in the stool.
Bone marrow tests
In patients with bone marrow disorders, medicines like Eltrombopag Polpharma may worsen these disorders. Changes in the bone marrow may be indicated by abnormal blood test results. The doctor may order direct bone marrow tests during treatment with Eltrombopag Polpharma.
Tests for gastrointestinal bleeding
If the patient is taking interferon-based treatment and Eltrombopag Polpharma, they will be monitored for signs of gastrointestinal bleeding after stopping treatment with Eltrombopag Polpharma.
Heart tests
The doctor may decide to perform a heart test on the patient during treatment with Eltrombopag Polpharma and perform an electrocardiogram (ECG).
Elderly patients (65 years and older)
There is limited data on the use of Eltrombopag Polpharma in patients aged 65 and older. Caution should be exercised when using Eltrombopag Polpharma in patients aged 65 and older.
Children and adolescents
Eltrombopag Polpharma is not recommended for children under 1 year of age with primary immune thrombocytopenia. The medicine is also not recommended for patients under 18 years of age with low platelet count associated with hepatitis C virus infection.
Eltrombopag Polpharma and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. This includes medicines available without a prescription and vitamins. Some commonly used medicines interact with Eltrombopag Polpharma- including both prescription and non-prescription medicines and products containing minerals. These include:
- antacids used to treat indigestion, heartburn, stomach ulcers(see also "When to take the medicine" in section 3)
- statins, which lower cholesterol levels
- certain medicines used to treat HIV infection, such as lopinavir and (or) ritonavir
- cyclosporin used in transplantsor immune system disorders
- mineral products, such as iron, calcium, magnesium, aluminum, selenium, and zinc, which may be components of vitamin and mineral supplements(see also "When to take the medicine" in section 3)
- medicines such as methotrexate and topotecan, used to treat cancer.
The patient should consult their doctor if they are taking any of the above medicines. Some of them should not be taken during treatment with Eltrombopag Polpharma, while others may require dose adjustment or appropriate timing of administration. The doctor will review the patient's medicines and recommend changes if necessary. If the patient is taking medicines to prevent blood clots, there is an increased risk of bleeding. The doctor will discuss this with the patient. If the patient is taking corticosteroids, danazol, and (or) azathioprine, the doses of these medicines may be reduced or their use may be stopped during concurrent use of Eltrombopag Polpharma.
Taking Eltrombopag Polpharma with food and drink
Eltrombopag Polpharma should not be taken with dairy products or drinks, as the calcium in dairy products affects the absorption of the medicine. For more information, see "When to take the medicine" in section 3.
Pregnancy and breastfeeding
Eltrombopag Polpharma should not be taken during pregnancy, unless the doctor recommends it. The effect of Eltrombopag Polpharma when taken during pregnancy is unknown.
- The patient should inform their doctor if they are pregnant, think they may be pregnant, or plan to have a baby.
- The patient should use appropriate contraceptionto prevent pregnancy while taking Eltrombopag Polpharma.
- The patient should inform their doctor if they become pregnant while taking Eltrombopag Polpharma.
Eltrombopag Polpharma should not be taken while breastfeeding. It is not known whether Eltrombopag Polpharma passes into breast milk. The patient should inform their doctor if they are breastfeedingor plan to breastfeed.
Driving and using machines
Eltrombopag Polpharma may cause dizzinessand other side effects that reduce attention. The patient should not drive or operate machineryunless they are sure that these symptoms do not occur.
Eltrombopag Polpharma contains isomalt and sodium
If the patient has previously been diagnosed with intolerance to some sugars, they should contact their doctor before taking the medicine. The medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means that the medicine is considered "sodium-free".
3. How to take Eltrombopag Polpharma
This medicine should always be taken exactly as prescribed by the doctor. If the patient has any doubts, they should consult their doctor or pharmacist. The patient should not change the dose or dosing schedule of Eltrombopag Polpharma unless their doctor or pharmacist recommends it. During treatment with Eltrombopag Polpharma, the patient will remain under the care of a doctor with experience in treating the patient's condition.
How much to take
In the case of primary immune thrombocytopenia
Adults and children(aged 6 to 17 years) - the usual starting dose in primary immune thrombocytopenia is one 50 mg tabletof Eltrombopag Polpharma per day. Patients of East Asian or Southeast Asian descent may require a lower starting dose of 25 mg.
Children(aged 1 to 5 years) - the usual starting dose in primary immune thrombocytopenia is one 25 mg tabletof Eltrombopag Polpharma per day.
In the case of hepatitis C
Adults- the usual starting dose in hepatitis C is one 25 mg tabletof Eltrombopag Polpharma per day. Patients of East Asian or Southeast Asian descent should start with the same dose of 25 mg.
The onset of action of Eltrombopag Polpharma may occur after 1 to 2 weeks. Depending on the patient's response to treatment with Eltrombopag Polpharma, the doctor may recommend a change in the daily dose.
How to take the tablets
The tablets should be swallowed whole with water.
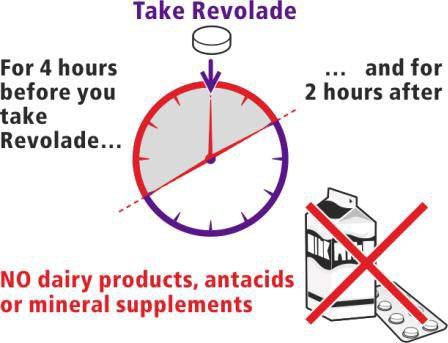
When to take the medicine
The patient should make sure that:
- for 4 hours beforetaking Eltrombopag Polpharma
- and for 2 hours aftertaking Eltrombopag Polpharma, the patient will notconsume the following foods:
- dairy products, such as cheese, butter, yogurt, ice cream
- milkor drinks containing milk, yogurt, or cream
- antacidsused to treat indigestion and heartburn
- vitamin and mineral supplementscontaining iron, calcium, magnesium, aluminum, selenium, and zinc.
If the patient does not follow these recommendations, Eltrombopag Polpharma will not be properly absorbed by the body.
The patient should consult their doctor for more information about suitable foods and drinks.
Taking more than the recommended dose of Eltrombopag Polpharma
The patient should contact their doctor or pharmacist immediately. If possible, they should show them the packaging of the medicine or this leaflet. The patient's condition will be monitored for any side effects and appropriate treatment will be administered promptly.
Missing a dose of Eltrombopag Polpharma
The patient should take the next dose at the usual time. The patient should not take more than one dose of Eltrombopag Polpharma per day.
Stopping treatment with Eltrombopag Polpharma
The patient should not stop taking Eltrombopag Polpharma without consulting their doctor. If the doctor recommends stopping treatment, the patient's platelet count will be monitored weekly for four weeks. See also "Bleeding or bruising after stopping treatment" in section 4. If the patient has any further doubts about taking this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Eltrombopag Polpharma can cause side effects, although not everybody gets them.
Symptoms to look out for: the patient should see a doctor
In patients taking Eltrombopag Polpharma for primary immune thrombocytopenia or low platelet count associated with hepatitis C, severe side effects may occur. It is essential to inform the doctor about these symptoms.
Increased risk of blood clots
In some patients, the risk of blood clots may increase, and medicines like Eltrombopag Polpharma may worsen this condition. Sudden blockage of a blood vessel by a blood clot is an uncommon side effect and may occur in up to 1 in 100 patients.
The patient should seek immediate medical attention if they experience any of the following symptoms of a blood clot:
- swelling, pain, warmth, redness, or tenderness in one leg
- sudden shortness of breath, especially with sudden chest pain or rapid breathing
- abdominal pain, abdominal enlargement, blood in the stool.
Liver disorders
Eltrombopag Polpharma may cause changes in blood test results that may indicate liver damage. Liver disorders (increased activity of enzymes in blood test results) are common and may occur in up to 1 in 10 patients. Other liver problems are uncommon and may occur in up to 1 in 100 patients.
If the patient experiences any of the following symptoms of liver disorders:
- yellowingof the skin or whites of the eyes (jaundice)
- abnormally dark urine
The patient should tell their doctor immediately.
Bleeding or bruising after stopping treatment
Usually, within two weeks of stopping treatment with Eltrombopag Polpharma, the patient's platelet count returns to the level before starting treatment with Eltrombopag Polpharma. A low platelet count may increase the risk of bleeding or bruising. The doctor will monitor the patient's platelet count for at least 4 weeks after stopping treatment with Eltrombopag Polpharma.
The patient should inform their doctorif they experience bruising or bleeding after stopping treatment with Eltrombopag Polpharma.
In some patients, gastrointestinal bleedingmay occur after stopping treatment with peginterferon, ribavirin, and Eltrombopag Polpharma. Symptoms include:
- black, tarry stools (a change in stool color, which is an uncommon side effect that may occur in up to 1 in 100 patients)
- blood in the stool
- vomiting blood or coffee-ground-like material.
The patient should tell their doctor immediatelyif they experience any of these symptoms.
The following side effects have been reported in adults with primary immune thrombocytopenia taking eltrombopag:
Very commonside effects (may occur in more than 1 in 10 patients):
- infection of the nose, sinuses, throat, and upper respiratory tract, upper respiratory tract infection
- cough, common cold
- nausea, diarrhea
- back pain.
Very commonside effects that may be detected in blood tests:
- increased activity of liver enzymes (alanine aminotransferase (ALT)).
Commonside effects (may occur in up to 1 in 10 patients):
- influenza, herpes, pneumonia, sinusitis, tonsillitis, pharyngitis, bronchitis, gastritis, gum bleeding, toothache, flu, oral herpes
- loss of appetite
- insomnia, depression
- numbness, tingling, prickling, or burning sensations, drowsiness, migraine
- eye disorders, including abnormal vision test results, dry eyes, eye pain, and blurred vision
- ear pain, dizziness
- pain, swelling, or tenderness in one leg (usually the calf) with warmth of the skin in the affected area (symptoms of deep vein thrombosis), hematoma, hot flashes
- runny nose
- mouth disorders, including dry mouth, mouth pain, tongue sensitivity, gum bleeding, mouth ulcers, toothache, vomiting, abdominal pain, bloating, flatulence
- abnormal liver function
- skin changes, including excessive sweating, rash, redness, itching, and flushing
- muscle pain, muscle spasms, muscle weakness, bone pain
- foamy urine
- heavy menstrual bleeding
- high fever, feeling hot, chest pain, feeling unwell.
Commonside effects that may be detected in blood tests:
- decreased red blood cell count (anemia), decreased platelet count (thrombocytopenia), decreased white blood cell count, decreased hemoglobin level, increased eosinophil count, increased white blood cell count (leukocytosis)
- increased uric acid level, decreased potassium level
- increased activity of liver enzymes (aspartate aminotransferase (AST)),
increased bilirubin level in the blood (a substance produced by the liver)
- increased levels of certain proteins, increased creatinine level
- increased alkaline phosphatase level.
Uncommonside effects (may occur in up to 1 in 100 patients):
- skin infection
- colon cancer
- allergic reaction
- loss of appetite, painful joint swelling due to gout
- lack of interest, mood changes, crying, which is hard to stop or occurs at an unexpected time
- balance problems, speech problems, and nerve problems, tremors, paralysis of one side of the body, migraine with aura, brain damage due to liver disease, numbness or tingling sensations in the hands or feet
- eye disorders, including increased tear production, cataracts, retinal bleeding
- rapid or irregular heartbeat, blue discoloration of the skin, cardiovascular disorders, which may be symptoms of heart and blood vessel problems, heart attack
- pain, swelling, or redness in the leg, which may be symptoms of blood clots, blood clots, redness
- sudden shortness of breath, especially with sudden chest pain and rapid breathing (see "Increased risk of blood clots" earlier in section 4), pulmonary embolism, blockage of a blood vessel in the lungs
- mouth disorders, including dry mouth, mouth pain, tongue sensitivity, gum bleeding, mouth ulcers, toothache, vomiting, abdominal pain, bloating, flatulence
- liver problems, including liver tumor, yellowing of the skin or whites of the eyes (jaundice), liver damage due to medication (see "Liver disorders" earlier in section 4)
- skin changes, including discoloration, peeling, redness, itching, and sweating, night sweats
- muscle weakness
- kidney problems, including kidney inflammation, frequent urination at night, kidney failure, white blood cells in the urine
- feeling hot, feeling unwell, bleeding around the catheter (if present) to the skin, redness or swelling around the wound, general feeling of being unwell, feeling of a foreign body
- sunburn.
Uncommonside effects that may be detected in laboratory tests:
- changes in the shape of red blood cells, decreased red blood cell count (anemia) due to excessive destruction of red blood cells (hemolytic anemia), increased myelocyte count, increased band neutrophil count, presence of immature white blood cells, which may indicate certain diseases, increased platelet count, increased hemoglobin level
- decreased calcium level
- increased urea level in the blood, increased protein level in the urine
- increased albumin level in the blood, increased total protein level, decreased albumin level in the blood, increased pH of the urine.
The following additional side effects have been reported in children (aged 1 to 17 years) with ITP taking eltrombopag:
If these side effects worsen, the patient should tell their doctor, pharmacist, or nurse.
Very commonside effects (may occur in more than 1 in 10 children):
- infection of the nose, sinuses, throat, and upper respiratory tract, common cold (upper respiratory tract infection)
- cough
- nausea, diarrhea, abdominal pain
- high fever.
Commonside effects (may occur in up to 1 in 10 children):
- sleep problems (insomnia)
- itching or runny nose, sore throat, rhinitis, sneezing, sore nose and throat
- toothache, mouth problems, including dry mouth, mouth pain, tongue sensitivity, gum bleeding, mouth ulcers.
The following side effects have been reported in patients with HCV taking eltrombopag in combination with peginterferon and ribavirin:
Very commonside effects (may occur in more than 1 in 10 patients):
- loss of appetite
- headache
- cough
- nausea, diarrhea
- itching, swelling of the hands or feet, unusual hair loss
- muscle pain, muscle weakness
- fever, feeling tired, flu-like illness, weakness, chills.
Very commonside effects that may be detected in blood tests:
- decreased red blood cell count (anemia).
Commonside effects (may occur in up to 1 in 10 patients):
- urinary tract infections, infection of the nose, sinuses, throat, and upper respiratory tract, common cold (upper respiratory tract infection), bronchitis, sinusitis, pharyngitis, flu-like illness, dry mouth, mouth pain or inflammation
- weight loss
- sleep problems, drowsiness, depression, anxiety
- dizziness, concentration and memory problems, mood changes, liver disease due to brain damage, numbness or tingling sensations in the hands or feet
- eye disorders, including increased tear production, cataracts, retinal bleeding
- dizziness
- rapid or irregular heartbeat
- shortness of breath, coughing up mucus, sore throat, and discomfort when swallowing
- gastrointestinal disorders, including vomiting, abdominal pain, indigestion, constipation, bloating, taste disorders, hemorrhoids, stomach pain or discomfort
- liver problems, including liver tumor, yellowing of the skin or whites of the eyes (jaundice), liver damage due to medication (see "Liver disorders" earlier in section 4)
- skin changes, including rash, dry skin, peeling, redness, itching, and sweating
- joint pain, back pain, bone pain, limb pain (arms, legs, hands, or feet), muscle spasms
- irritability, general feeling of being unwell, skin reaction, such as redness or swelling and pain at the injection site, chest pain, and discomfort
- depression, anxiety, sleep problems, nervousness.
- fever, headache.
Commonside effects that may be detected in blood tests:
- increased glucose (sugar) level in the blood, decreased white blood cell count, decreased neutrophil count, decreased albumin level in the blood, decreased hemoglobin level, increased bilirubin level in the blood (a substance produced by the liver), changes in blood clotting enzymes.
Uncommonside effects (may occur in up to 1 in 100 patients):
- stomach flu (gastroenteritis), sore throat
- decreased red blood cell count (anemia) due to excessive destruction of red blood cells (hemolytic anemia)
- confusion, agitation
- mouth ulcers, gastritis
- blood clots in the vein that carries blood to the liver (which may cause liver damage and (or) gastrointestinal problems), liver failure
- skin changes, including discoloration, peeling, redness, itching, and sweating, night sweats
- abnormal blood clotting in small blood vessels with kidney failure, painful urination
- rash, bruising at the injection site, chest discomfort
- heart rhythm problems (prolonged QT interval).
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Eltrombopag Polpharma
The medicine should be stored out of sight and reach of children. The patient should not take this medicine after the expiry date stated on the packaging and blister after EXP. The expiry date refers to the last day of the month. The inscription on the packaging after the abbreviation EXP indicates the expiry date, and after the abbreviation Lot indicates the batch number. There are no special precautions for storing the medicine. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Eltrombopag Polpharma contains
- The active substance of the medicine is eltrombopag olamine. Eltrombopag Polpharma 12.5 mg: each film-coated tablet contains eltrombopag olamine equivalent to 12.5 mg of eltrombopag. Eltrombopag Polpharma 25 mg: each film-coated tablet contains eltrombopag olamine equivalent to 25 mg of eltrombopag. Eltrombopag Polpharma 50 mg: each film-coated tablet contains eltrombopag olamine equivalent to 50 mg of eltrombopag. Eltrombopag Polpharma 75 mg: each film-coated tablet contains eltrombopag olamine equivalent to 75 mg of eltrombopag.
- Other ingredients are: microcrystalline cellulose, mannitol, povidone, isomalt (E 953), calcium silicate, sodium carboxymethylcellulose (type A), magnesium stearate (tablet core); hypromellose, titanium dioxide (E 171), iron oxide red (E 172), iron oxide yellow (E 172) (only in 12.5 mg, 25 mg, and 50 mg doses), triacetin (tablet coating).
What Eltrombopag Polpharma looks like and contents of the pack
Eltrombopag Polpharma 12.5 mg is orange to brown, round, biconvex, film-coated tablets with the inscription "I" on one side and a diameter of about 5.5 mm.
Eltrombopag Polpharma 25 mg is dark pink, round, biconvex, film-coated tablets with the inscription "II" on one side and a diameter of about 8 mm.
Eltrombopag Polpharma 50 mg is pink, round, biconvex, film-coated tablets with the inscription "III" on one side and a diameter of about 10 mm.
Eltrombopag Polpharma 75 mg is red to brown, round, biconvex, film-coated tablets with the inscription "IV" on one side and a diameter of about 12 mm.
Eltrombopag Polpharma 12.5 mg is available in cardboard boxes containing 10, 14, 28, 30, or 84 film-coated tablets packaged in blisters or in cardboard boxes containing 10x1, 14x1, 28x1, 30x1, or 84x1 film-coated tablets packaged in single-dose perforated blisters.
Eltrombopag Polpharma 25 mg is available in cardboard boxes containing 10, 14, 28, 30, or 84 film-coated tablets packaged in blisters or in cardboard boxes containing 10x1, 14x1, 28x1, 30x1, or 84x1 film-coated tablets packaged in single-dose perforated blisters.
Eltrombopag Polpharma 50 mg is available in cardboard boxes containing 10, 14, 28, 30, or 84 film-coated tablets packaged in blisters or in cardboard boxes containing 10x1, 14x1, 28x1, 30x1, or 84x1 film-coated tablets packaged in single-dose perforated blisters.
Eltrombopag Polpharma 75 mg is available in cardboard boxes containing 10, 14, 28, 30, or 84 film-coated tablets packaged in blisters or in cardboard boxes containing 10x1, 14x1, 28x1, 30x1, or 84x1 film-coated tablets packaged in single-dose perforated blisters.
Not all pack sizes may be marketed.
Marketing authorization holder
Polpharma S.A., ul. Pelplińska 19, 83-200 Starogard Gdański, tel. +48 22 364 61 01
Manufacturer
Synthon Hispania S.L., Calle De Castello 1, 08330 Sant Boi De Llobregat, Spain
Synthon B.V., Microweg 22, 6545 CM Nijmegen, Netherlands
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterSynthon B.V. Synthon Hispania S.L.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Eltrombopag PolpharmaDosage form: Tablets, 25 mgActive substance: eltrombopagPrescription requiredDosage form: Tablets, 50 mgActive substance: eltrombopagPrescription requiredDosage form: Tablets, 25 mgActive substance: eltrombopagPrescription required
Alternatives to Eltrombopag Polpharma in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Eltrombopag Polpharma in Spain
Alternative to Eltrombopag Polpharma in Ukraine
Online doctors for Eltrombopag Polpharma
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Eltrombopag Polpharma – subject to medical assessment and local rules.














