
Egoropal

Ask a doctor about a prescription for Egoropal

How to use Egoropal
Leaflet accompanying the packaging: information for the user
Egoropal, 25 mg, prolonged-release suspension for injection
In a pre-filled syringe
Egoropal, 50 mg, prolonged-release suspension for injection in a pre-filled syringe
Egoropal, 75 mg, prolonged-release suspension for injection in a pre-filled syringe
Egoropal, 100 mg, prolonged-release suspension for injection in a pre-filled syringe
Egoropal, 150 mg, prolonged-release suspension for injection in a pre-filled syringe
Paliperidone
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Egoropal and what is it used for
- 2. Important information before using Egoropal
- 3. How to use Egoropal
- 4. Possible side effects
- 5. How to store Egoropal
- 6. Contents of the packaging and other information
1. What is Egoropal and what is it used for
Egoropal contains the active substance paliperidone, which belongs to a group of antipsychotic medicines and is used to treat schizophrenia in adult patients who have been stabilized on paliperidone or risperidone.
If the patient has previously responded to paliperidone or risperidone and has mild or moderate symptoms, the doctor may start treatment with Egoropal without prior stabilization on paliperidone or risperidone.
Schizophrenia is a disease with "positive" and "negative" symptoms. Positive symptoms mean the accumulation of symptoms that usually do not occur. A person with schizophrenia may, for example, hear voices or see things that do not exist (these are hallucinations), believe in untrue things (these are delusions), or be overly suspicious of others. Negative symptoms mean the lack of usually occurring behaviors or feelings. A person with schizophrenia may, for example, seem withdrawn, closed off, and not respond emotionally at all, or may have difficulty speaking clearly and logically. People with this disease may also experience depression, anxiety, guilt, or tension.
Egoropal may help alleviate the symptoms of the disease and prevent their recurrence.
2. Important information before using Egoropal
When not to use Egoropal
- if the patient is allergic to paliperidone or any of the other ingredients of this medicine (listed in section 6);
- if the patient is allergic to another antipsychotic medicine, including the active substance risperidone.
Warnings and precautions
Before starting treatment with Egoropal, discuss it with your doctor, pharmacist, or nurse.
The use of this medicine has not been studied in elderly patients with dementia. However, in such patients treated with other similar medicines, there may be an increased risk of stroke or death (see section 4, Possible side effects).
All medicines can cause side effects. Some side effects of this medicine may worsen the symptoms of other medical conditions. Therefore, during treatment with this medicine, discuss with your doctor any of the following conditions that may potentially worsen:
- if the patient has Parkinson's disease
- if the patient has ever been diagnosed with a condition whose symptoms include high fever and muscle stiffness (also known as malignant neuroleptic syndrome)
- if the patient has ever experienced abnormal movements of the tongue or facial muscles (tardive dyskinesia)
- if the patient has had a low white blood cell count in the past (which may or may not have been caused by the action of other medicines)
- if the patient has diabetes or is prone to it
- if the patient has breast cancer or a pituitary tumor
- if the patient has heart disease or is being treated for heart disease, which predisposes to low blood pressure
- if the patient has low blood pressure when changing position from lying down to standing or sitting
- if the patient has epilepsy
- if the patient has kidney function disorders
- if the patient has liver function disorders
- if the patient experiences prolonged or painful erections
- if the patient has a problem with body temperature regulation or overheating
- if the patient has abnormal, elevated levels of the hormone prolactin in the blood or suspected prolactin-dependent tumor
- if the patient or their family members have had cases of blood clots in blood vessels, as antipsychotic medicines are associated with their formation.
If the patient has any of the above conditions, they should consult their doctor, who may adjust the dose or periodically monitor the patient.
The doctor may order a white blood cell count test, as very rarely, patients taking this medicine have had a dangerously low number of a certain type of white blood cell necessary for fighting infections.
Even if the patient has previously tolerated oral paliperidone or risperidone, rare allergic reactions may occur after injections of paliperidone. Medical help should be sought immediately if the patient experiences a rash, throat swelling, itching, or difficulty breathing, as these may be symptoms of a severe allergic reaction.
This medicine may cause weight gain. Significant weight gain can have a negative impact on the patient's health. The doctor will regularly check the patient's weight.
The doctor will check if the patient has symptoms of high blood sugar, as patients taking this medicine have had new cases of diabetes or worsening of existing diabetes. In patients with existing diabetes, blood sugar levels should be regularly checked.
Because this medicine can suppress vomiting, it may mask the body's normal response to swallowing toxic substances or other medical conditions.
During eye surgery for cataracts, the pupil (the black part of the eye) may not dilate sufficiently. The iris (the colored part of the eye) may also be flaccid during the procedure, which can result in eye damage. If the patient is scheduled for eye surgery, they should tell their ophthalmologist about taking this medicine.
Children and adolescents
This medicine is not used in people under 18 years of age.
Egoropal and other medicines
Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Taking this medicine with carbamazepine (an antiepileptic and mood stabilizer) may require a change in the dose of this medicine.
Due to the fact that this medicine acts primarily in the brain, interactions with other medicines such as other psychotropic medicines, opioids, antihistamines, and sedatives that act in the brain may cause an increase in side effects, such as drowsiness, or other effects on the brain.
This medicine may cause a decrease in blood pressure, so caution should be exercised when taking this medicine with other medicines that lower blood pressure.
This medicine may weaken the effect of medicines used to treat Parkinson's disease and restless legs syndrome (e.g., levodopa).
This medicine may cause abnormalities in the electrocardiogram (ECG) characterized by prolongation of the time it takes for the electrical impulse to pass through a certain part of the heart (called "prolongation of the QT interval"). Other medicines with this effect include those used to regulate heart rhythm or treat infections, as well as other antipsychotic medicines.
If the patient has a tendency to seizures, this medicine may increase the risk of their occurrence. Other medicines with this effect include certain medicines used to treat depression or infections, as well as other antipsychotic medicines.
Care should be taken when using Egoropal with medicines that increase the activity of the central nervous system (psychostimulant medicines such as methylphenidate).
Using Egoropal with alcohol
Alcohol should be avoided.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
This medicine should not be used during pregnancy, unless discussed with a doctor.
In newborns whose mothers used paliperidone in the last trimester (last 3 months of pregnancy), the following symptoms may occur: trembling, muscle stiffness, and/or weakness, drowsiness, agitation, difficulty breathing, and difficulty feeding. If such symptoms are observed in the patient's child, they should contact their doctor.
This medicine may pass from the mother's body into her breast milk and harm the baby.
Therefore, breastfeeding should be avoided while using this medicine.
Driving and operating machinery
During treatment with this medicine, dizziness, extreme fatigue, and vision disturbances (see section 4) may occur. This should be taken into account in situations where full alertness is required, such as driving a vehicle or operating machinery.
Egoropal contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which means that the medicine is considered "sodium-free".
3. How to use Egoropal
This medicine is administered by a doctor or other medical staff. The doctor will inform the patient when to administer the next injection. It is essential not to miss a scheduled dose.
If it is not possible to keep the appointment with the doctor, they should be contacted immediately to schedule another appointment as soon as possible.
The patient will receive the first (150 mg) and second (100 mg) injections of this medicine in the upper arm with an approximate 1-week interval. Then, the patient will receive an injection (in a dose ranging from 25 mg to 150 mg) in the upper arm or buttock once a month.
If the doctor decides to switch from using long-acting risperidone injections or other long-acting paliperidone injections to this medicine, the first injection of this medicine (in a dose ranging from 25 mg to 150 mg) will be administered in the upper arm or buttock on the day of the next scheduled injection. Then, an injection (in a dose ranging from 25 mg to 150 mg) will be administered in the upper arm or buttock once a month.
Depending on the symptoms occurring in the patient, the doctor may increase or decrease the administered dose of the medicine by one level of strength during the scheduled monthly injection.
Patient with kidney function disorders
The doctor will adjust the dose of the medicine, taking into account the kidney function. If the patient has mild kidney function disorders, the doctor may decrease the dose. This medicine should not be used in cases of moderate or severe kidney function disorders.
Elderly patients
The doctor may decrease the dose of the medicine if the patient has impaired kidney function.
Using a higher dose of Egoropal than recommended
This medicine will be administered under medical supervision, so administering too high a dose is unlikely.
In patients who received too high a dose of paliperidone, the following symptoms may occur: drowsiness and sedation, rapid heart rate, low blood pressure, abnormal ECG (electrocardiogram), or slow or abnormal movements of facial, body, arm, or leg muscles.
Stopping the use of Egoropal
If the patient stops taking the injections, the medicine will stop working. The use of the medicine should not be stopped unless the doctor decides to do so, as the symptoms of the disease may return.
In case of any further doubts about the use of this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Inform your doctor immediately if:
- the patient experiences blood clots in the veins, especially in the legs (symptoms include swelling, pain, and redness of the leg), which can travel through the blood vessels to the lungs, causing chest pain and difficulty breathing. If such symptoms occur, medical help should be sought immediately.
- the patient with dementia experiences a sudden change in mental state or sudden weakness or numbness of the face, arms, or legs, especially one-sided, or speech disturbances, even for a short time. These symptoms may indicate a stroke.
- the patient experiences fever, muscle stiffness, sweating, or decreased level of consciousness (a condition called malignant neuroleptic syndrome). Immediate treatment may be necessary.
- a man experiences prolonged or painful erections. This condition is called priapism. Immediate treatment may be necessary.
- the patient experiences involuntary rhythmic movements of the tongue, lips, or face. It may be necessary to discontinue treatment with paliperidone.
- the patient experiences a severe allergic reaction characterized by fever, swelling of the lips, face, tongue, or throat, difficulty breathing, itching, skin rash, and sometimes a drop in blood pressure (called anaphylactic reaction). Even if the patient has previously tolerated risperidone or paliperidone orally, rare allergic reactions may occur after injections of paliperidone.
- the patient is scheduled for eye surgery, they should inform their ophthalmologist about taking this medicine. During eye surgery for cataracts, the iris (the colored part of the eye) may become flaccid (a condition called "floppy iris syndrome"), which can lead to eye damage.
- the patient has a dangerously low number of certain white blood cells necessary for fighting infections.
The following side effects may occur:
Very common side effects: may occur in more than 1 in 10 patients
- difficulty falling asleep or maintaining sleep continuity.
Common side effects: may occur in less than 1 in 10 patients
- cold symptoms, urinary tract infections, flu-like symptoms
- Egoropal may increase the level of the hormone prolactin in the blood (which may or may not cause symptoms). If symptoms of elevated prolactin levels occur, they may include in men: breast swelling, difficulty achieving or maintaining an erection, or other sexual disorders. In women, they may include discomfort in the breast area, milk leakage from the breasts, absence of menstrual bleeding, or other menstrual cycle disorders
- high blood sugar, weight gain, weight loss, decreased appetite
- irritability, depression, anxiety
- parkinsonism: this condition may include slow or abnormal movements, a feeling of stiffness or muscle tension (which makes the patient's movements uneven, jerky), and sometimes even a feeling of "freezing" of movements, followed by unlocking. Other symptoms of parkinsonism include a slow, shuffling gait, tremors at rest, increased saliva production, and a mask-like face
- psychomotor agitation, feeling of drowsiness or decreased alertness
- dystonia: this condition includes slow or sustained involuntary muscle contractions. Although it can affect any part of the body (which can result in an abnormal posture), dystonia most commonly affects the facial muscles, including abnormal movements of the eyes, lips, tongue, or jaw
- dizziness
- dyskinesia: this condition includes involuntary muscle movements and can take the form of repetitive, spasmodic, or twisting movements or jerks
- tremors
- headaches
- rapid heart rate
- high blood pressure
- cough, stuffy nose
- abdominal pain, vomiting, nausea, constipation, diarrhea, indigestion, toothache
- increased liver enzyme activity in the blood
- bone or muscle pain, back pain, joint pain
- absence of menstruation
- fever, weakness, fatigue
- reaction at the injection site, including itching, pain, or swelling.
Uncommon side effects: may occur in less than 1 in 100 patients
- pneumonia, bronchitis, respiratory tract infections, sinusitis, urinary tract infections, ear infections, fungal nail infections, tonsillitis, skin infections
- decreased white blood cell count, decreased number of certain white blood cells that protect against infections, anemia
- allergic reaction
- onset of diabetes and worsening of existing diabetes, increased insulin levels in the blood (a hormone that regulates blood sugar levels)
- increased appetite
- loss of appetite leading to malnutrition and low body weight
- high levels of triglycerides (fats) in the blood, increased cholesterol levels in the blood
- sleep disturbances, elevated mood (mania), decreased libido, nervousness, nightmares
- late dyskinesia (involuntary, rhythmic movements of the face, tongue, or other parts of the body). If the patient experiences involuntary, rhythmic movements of the tongue, lips, or face, they should immediately inform their doctor. It may be necessary to discontinue treatment with this medicine
- fainting, need to move limbs, dizziness when changing position from lying down to standing, concentration disorders, speech difficulties, loss of taste, decreased sensitivity to pain and touch on the skin, feeling of tingling, prickling, or numbness of the skin
- blurred vision, eye infection or conjunctivitis, dry eye
- vertigo, ringing in the ears, ear pain
- heart block, abnormal conduction of electrical impulses in the heart, prolongation of the QT interval in the heart, rapid heart rate when changing position from lying down to standing, slow heart rate, abnormal ECG, palpitations
- low blood pressure, low blood pressure when changing position from lying down to standing (which may cause some patients taking this medicine to faint, feel dizzy, or lose consciousness when standing up or getting up)
- shallow breathing, sore throat, nosebleeds
- abdominal discomfort, stomach or intestinal infection, difficulty swallowing, dry mouth
- excessive gas
- increased activity of the enzyme GGTP (a liver enzyme - gamma-glutamyltransferase) in the blood, increased liver enzyme activity in the blood
Increased activity of the enzyme CPK (creatine phosphokinase) in the blood, an enzyme that is sometimes released from damaged muscles
- hives, itching, rash, hair loss, eczema, dry skin, redness of the skin, acne, subcutaneous abscess
- muscle cramps, joint stiffness, muscle weakness
- urinary incontinence, frequent urination, painful urination
- erectile dysfunction, ejaculation disorders, absence of menstruation, and other menstrual cycle disorders (in women), breast enlargement in men, sexual disorders, breast pain, galactorrhea
- swelling of the face, lips, eyes, or tongue, swelling of the body, arms, or legs
- increased body temperature
- change in gait
- chest pain, chest discomfort, malaise
- scleroderma
- falls
Rare side effects: may occur in less than 1 in 1,000 patients
- eye infection
- skin rash caused by mites, scaling, and itching of the scalp or body
- increased number of eosinophils (a type of white blood cell) in the blood
- decreased platelet count (blood cells responsible for stopping bleeding)
- head tremors
- abnormal secretion of the hormone that regulates the amount of urine
- sugar in the urine
- life-threatening complications of untreated diabetes
- low blood sugar
- excessive thirst
- lack of movement and reaction to stimuli in a patient who is not sleeping (catatonia)
- confusion
- sleepwalking (walking while sleeping)
- apathy
- inability to achieve orgasm
- severe allergic reaction with swelling, which may involve the throat and lead to difficulty breathing
- skin discoloration
- abnormal body posture
- in newborns whose mothers used Egoropal during pregnancy, side effects of the medicine and/or withdrawal symptoms may occur, such as irritability, slow or sustained muscle contractions, tremors, drowsiness, agitation, difficulty breathing, and difficulty feeding
- low body temperature
- necrosis at the injection site and ulcer at the injection site
Side effects with unknown frequency: cannot be estimated from the available data
- dangerously low number of certain white blood cells responsible for fighting infections
- severe allergic reaction characterized by fever, swelling of the lips, face, tongue, or throat, difficulty breathing, itching, skin rash, and sometimes a drop in blood pressure
- dangerously excessive water intake - water intoxication
- eating disorders related to sleep
- coma due to uncontrolled diabetes
- decreased oxygenation of various parts of the body (due to decreased blood flow)
- rapid, shallow breathing, pneumonia caused by inhalation of food, voice disorders
- lack of intestinal peristalsis resulting in obstruction
- jaundice (yellowing of the skin and eyes)
- severe or life-threatening rash with blisters and peeling skin, which may appear in the mouth, nose, eyes, and genitals, as well as around these areas, and may also spread to other parts of the body (Stevens-Johnson syndrome or toxic epidermal necrolysis)
- severe allergic reaction with swelling, which may involve the throat and lead to difficulty breathing
- skin discoloration
- abnormal body posture
Reporting side effects
If side effects occur, including any not listed in this leaflet, the patient should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects can help gather more information on the safety of the medicine.
5. How to store Egoropal
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and pre-filled syringe.
The expiry date refers to the last day of the month.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Egoropal contains
- The active substance of the medicine is paliperidone. Each pre-filled syringe of Egoropal 25 mg contains 39 mg of paliperidone palmitate. Each pre-filled syringe of Egoropal 50 mg contains 78 mg of paliperidone palmitate. Each pre-filled syringe of Egoropal 75 mg contains 117 mg of paliperidone palmitate. Each pre-filled syringe of Egoropal 100 mg contains 156 mg of paliperidone palmitate. Each pre-filled syringe of Egoropal 150 mg contains 234 mg of paliperidone palmitate.
- The other ingredients are: Polysorbate 20 Macrogol 4000 Citric acid monohydrate Disodium phosphate Sodium dihydrogen phosphate monohydrate Sodium hydroxide (to adjust pH) Water for injections
What Egoropal looks like and contents of the pack
Egoropal is a white or off-white prolonged-release suspension for injection in a pre-filled syringe.
Each carton contains 1 pre-filled syringe and 2 needles.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Egis Pharmaceuticals PLC
Keresztúri út 30-38
1106 Budapest
Hungary
Manufacturer
PHARMATHEN INTERNATIONAL S.A
Industrial Park Sapes
Rodopi Prefecture, Block No 5
69300 Rodopi
Greece
Pharmathen S.A
Dervenakion 6
15351 Pallini Attiki
Greece
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Denmark
Egoropal
Poland
Egoropal
Hungary
Egoropal 25mg, 50mg, 75mg, 100mg, 150mg, retardszuszpenziós injekció előretöltött fecskendőben
Czech Republic
Egoropal
Bulgaria
Egoropal 25mg, 50mg, 75mg, 100mg, 150mg, инжекционна суспензия с удължено освобождаване в предварително напълнена спринцовка
Egoropal 25mg, 50mg, 75mg, 100mg, 150mg, prolonged-release suspension for injection
Lithuania
Egoropal 25mg, 50mg, 75mg, 100mg, 150mg, pailginto atpalaidavimo injekcinė suspensija užpildytame švirkšte
Latvia
Egoropal 25mg, 50mg, 75mg, 100mg, 150mg, ilgstošas darbības suspensija injekcijām pilnšļircē
Slovakia
Egoropal 25mg, 50mg, 75mg, 100mg, 150mg, injekčná suspenzia s predĺženým uvoľňovaním v naplnenej injekčnej striekačke
For more detailed information, please contact the representative of the marketing authorization holder.
EGIS Polska sp. z o.o.:
Komitetu Obrony Robotników 45D
02-146 Warsaw
Phone: +48 22 417 92 00
Date of last revision of the leaflet:16.12.2023
Information intended for healthcare professionals only and should be read in conjunction with the full product information (Summary of Product Characteristics).
The suspension for injection is for single use only. Before administration, check for any foreign particles in the suspension. If any foreign particles are visible in the pre-filled syringe, do not use the medicinal product.
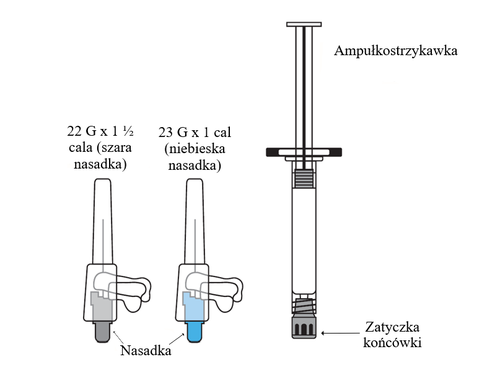
The packaging contains a pre-filled syringe and 2 needles with a safety device [22G 1.5 inches (38.1 mm x 0.72 mm) and 23G 1 inch (25.4 mm x 0.64 mm)] for intramuscular injection.
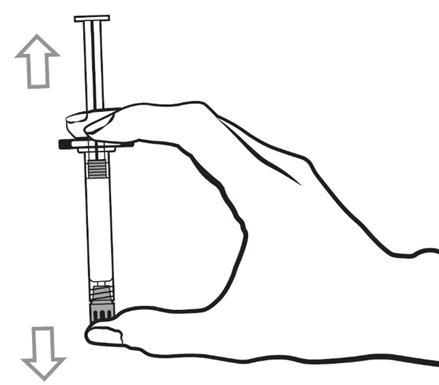
- 1. Shake the pre-filled syringe vigorously for at least 10 seconds to ensure the suspension is homogeneous.
- 2. Choose the appropriate needle.
The first initiating dose of the medicinal product Egoropal (150 mg) should be administered on Day 1 in the DELTOID MUSCLE using the deltoid injection needle. The second initiating dose of the medicinal product Egoropal (100 mg) should be administered in the DELTOID MUSCLE one week later (Day 8), using the deltoid injection needle.
In case of switching from risperidone long-acting injections or other paliperidone long-acting injections to this medicinal product, the first injection of this medicinal product (in a dose ranging from 25 mg to 150 mg) can be administered in the DELTOID MUSCLE or GLUTEAL MUSCLE on the day of the next scheduled injection, using the appropriate needle. Monthly maintenance doses can be administered in the DELTOID MUSCLE or GLUTEAL MUSCLE using the appropriate needle.
For deltoid muscle injections in patients with a body weight of less than 90 kg, use the 1-inch, 23G (25.4 mm x 0.64 mm) needle (needle with a blue hub). If the patient's body weight is 90 kg or more, use the 1.5-inch, 22G (38.1 mm x 0.72 mm) needle (needle with a gray hub).
For gluteal muscle injections, use the 1.5-inch, 22G (38.1 mm x 0.72 mm) needle (needle with a gray hub).
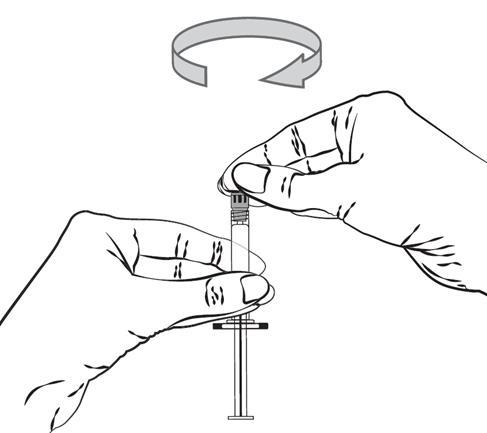
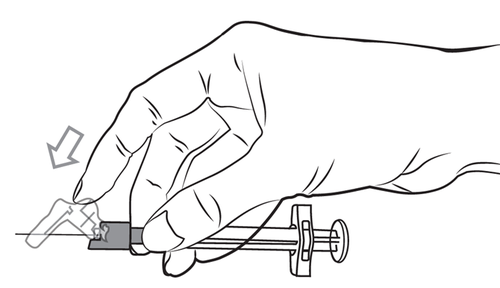
- 3. Hold the pre-filled syringe with the needle shield facing upwards, and remove the rubber needle shield by a gentle twisting motion.
- 4. Tear open the outer packaging of the needle with a safety device. Grasp the needle shield by the plastic packaging. Hold the pre-filled syringe with the needle facing upwards. Mount the needle with a safety device onto the pre-filled syringe by gently screwing, taking care not to touch the needle. Before administration, always check for any signs of damage or leakage.
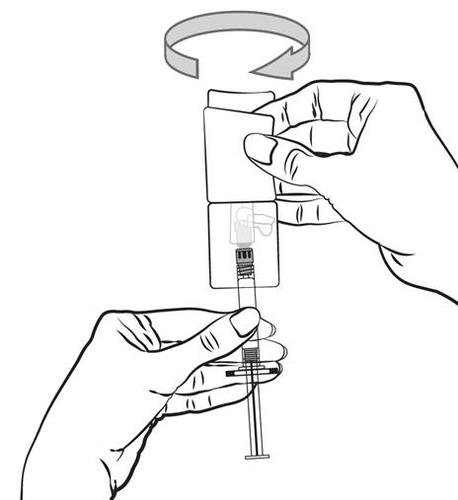
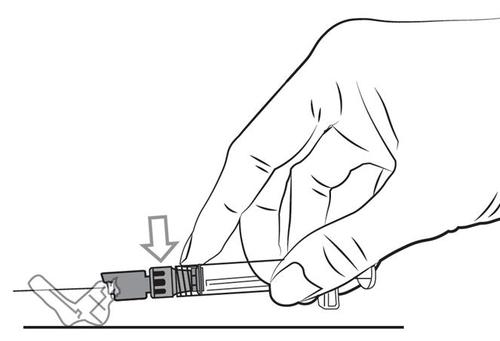
- 5. Remove the needle shield by a simple pull in a straight line. Do not twist the needle shield, as this may cause the needle to loosen from the pre-filled syringe.
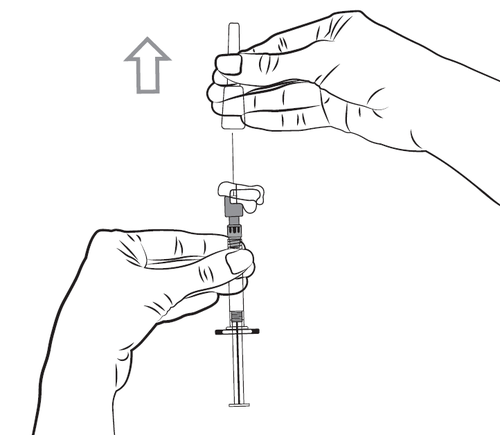
- 6. Hold the pre-filled syringe with the needle facing upwards to remove any air bubbles. Expel any air by slowly pushing the plunger forward.
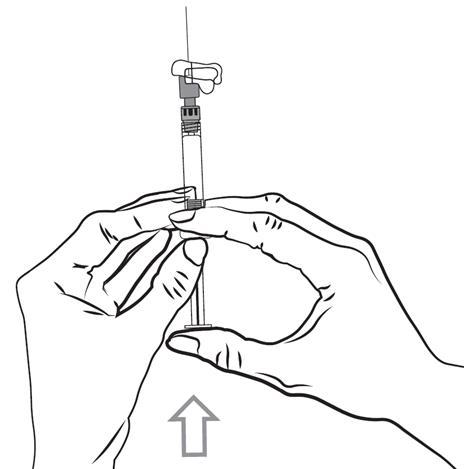
- 7. Administer the entire contents of the pre-filled syringe intramuscularly, slowly, and deeply into the selected muscle of the patient - deltoid or gluteal. Do not administer intravenously or subcutaneously.
- 8. After completing the injection, use the thumb or finger of one hand (8a, 8b) or a flat surface (8c) to activate the needle safety device. The clicking sound confirms that the needle is properly secured. Dispose of the pre-filled syringe with the needle in an approved sharps container.
8a
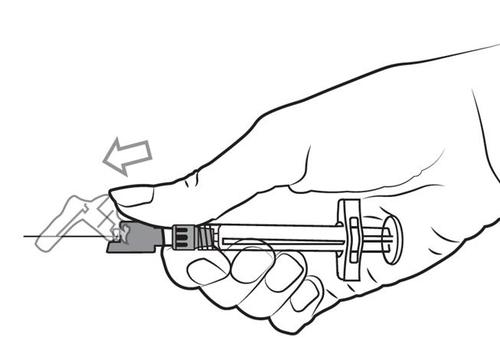
8b
8c
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterPharmaten International S.A. Pharmaten S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EgoropalDosage form: Suspension, 25 mgActive substance: paliperidoneManufacturer: Pharmathen International S.A. Pharmathen S.A.Prescription requiredDosage form: Suspension, 75 mgActive substance: paliperidoneManufacturer: Pharmaten International S.A. Pharmaten S.A.Prescription requiredDosage form: Suspension, 100 mgActive substance: paliperidoneManufacturer: Pharmaten S.A. Pharmathen International S.A.Prescription required
Alternatives to Egoropal in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Egoropal in Spain
Alternative to Egoropal in Ukraine
Online doctors for Egoropal
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Egoropal – subject to medical assessment and local rules.









