
Disport

Ask a doctor about a prescription for Disport

How to use Disport
PATIENT INFORMATION LEAFLET: USER INFORMATION
Dysport
300 units; 500 units of Clostridium botulinum type A neurotoxin complex,
powder for solution for injection
You should carefully read the contents of this leaflet before using the medicine, as it contains important information for the patient.
You should keep this leaflet so that you can read it again if you need to.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Dysport and what is it used for
- 2. Important information before using Dysport
- 3. How to use Dysport
- 4. Possible side effects
- 5. How to store Dysport
- 6. Package contents and other information
1. What is Dysport and what is it used for
Dysport contains Clostridium botulinum type A neurotoxin complex (commonly known as botulinum toxin) produced by the bacterium Clostridium botulinum (botulinum toxin rod). Its action is based on inhibiting muscle contraction by preventing the release of a chemical substance that causes physiological muscle contraction. This helps to reduce abnormal muscle tension called muscle spasticity.
Indications for use
- Symptomatic treatment of focal spasticity of the lower limbs in children with cerebral palsy aged 2 years or older
- Symptomatic treatment of focal spasticity of the upper limbs in children with cerebral palsy aged 2 years or older
- Urinary incontinence (involuntary urination) due to problems with the urinary bladder related to spinal cord damage or multiple sclerosis in patients who regularly undergo clean intermittent catheterization
- Cervical dystonia in adults
- Blepharospasm in adults
- Hemifacial spasm in adults
- Upper limb spasticity in adults
- Lower limb spasticity in adults
- Excessive axillary sweating
2. Important information before using Dysport
When not to use Dysport
Warnings and precautions
Before starting treatment with Dysport, you should discuss this with your doctor:
Dysport and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Some medicines that affect nerve-muscle function may enhance the effect of Dysport.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Dysport is not recommended for use during pregnancy, unless it is clearly necessary.
Dysport is not recommended for use in breastfeeding women.
Children and adolescents
In the treatment of spasticity associated with cerebral palsy, Dysport should be used in children aged 2 years or older.
Driving and using machines
After injection of Dysport, transient muscle weakness and vision disturbances may occur. In such cases, you should not drive vehicles or operate machines.
This medicine contains human albumin, a type of protein derived from human blood.
The risk of transmitting infectious agents is extremely low, but it cannot be completely ruled out.
3. How to use Dysport
The injection will be performed by a doctor who will decide how often the medicine should be administered. This will depend on the type of symptoms found in the patient.
Dysport should only be used for one patient and only during one therapeutic session.
In the treatment of symptomatic focal spasticity of the lower limbs in children:Children aged 2 years or older: the dose is determined by the doctor. Dysport is injected into the muscles of the lower limbs affected by spasticity.
The total dose of Dysport per therapeutic session should not exceed 1000 units or 30 units/kg. Injections will be performed approximately every 16-22 weeks or as needed, but no more frequently than every 12 weeks.
In the treatment of symptomatic focal spasticity of the upper limbs in children:Children aged 2 years or older: the dose is determined by the doctor. Dysport is injected into the muscles of the upper limb affected by spasticity. In the case of injection into one upper limb, the dose during one therapeutic session should not exceed 640 units or 16 units/kg, whichever is smaller. In the case of injection into both upper limbs during one therapeutic session, the dose should not exceed 840 units or 21 units/kg, whichever is smaller. Usually, within a few weeks after the end of treatment, muscle spasms should subside, and the effect of treatment should last for about 16-28 weeks. The doctor will repeat the procedures approximately every 16-28 weeks or as needed, but no more frequently than every 16 weeks.
In the simultaneous treatment of focal spasticity of the upper and lower limbs in children:If it is necessary to treat both the upper and lower limbs during the same therapeutic session, the doctor should determine the dose of Dysport to be injected into each limb, not exceeding the total dose administered during one therapeutic session of 1000 units or 30 units/kg, whichever is smaller. Re-treatment of both the upper and lower limbs should be considered no earlier than 12-16 weeks after the previous therapeutic session. The optimal time for re-treatment should be determined based on the progression of the disease and the response to treatment.
In the treatment of urinary incontinence:The first dose administered into the urinary bladder muscle is 600 units, but during subsequent injections, the doctor may decide to increase the dose to 800 units.
Dysport will be administered during cystoscopy. An endoscope, equipped with a light source at the end, will be inserted into the urinary bladder through the opening through which urine is excreted (called the urethral orifice). This will allow the doctor performing the procedure to have insight into the inside of the urinary bladder, enabling the injection of Dysport into the urinary bladder wall. Dysport will only be administered to patients who undergo clean intermittent catheterization (CIC). CIC is a procedure during which a catheter (a soft, hollow tube inserted into the urethra to facilitate the removal of urine from the urinary bladder) is temporarily inserted into the urinary bladder and then removed after the bladder has been emptied. For further details on the procedure, you should consult your doctor.
In order to prevent urinary tract infections, it will be necessary to take antibiotics. If you are taking a blood-thinning medicine, your doctor will modify the treatment before and after the injection with Dysport. Before the injection, you may receive local or general anesthesia or a sedative. You will be monitored for at least 30 minutes after the injection. Usually, symptoms should subside within 2 weeks, and improvement may last up to 48 weeks. Your doctor will decide whether to repeat the treatment if necessary, but no more frequently than every 12 weeks.
In the treatment of cervical dystonia:Typically, the first dose of Dysport is 500 units. Your doctor may divide this dose, injecting it into several places on the neck, presumably into 2 or 3 of the most active muscles.
Patients with significant underweight or elderly individuals may be given a smaller dose.
Relief of muscle spasms should usually occur within 1 week. Depending on how long the effect of treatment lasts, additional doses (250-1000 units) can be administered approximately every 12-16 weeks.
In the treatment of blepharospasm and hemifacial spasm:Your doctor will perform injections in the facial area affected by the disease. The first dose is 40 units per eye. Depending on how long the effect of treatment lasts, injections can be performed approximately every 12 weeks. If a longer duration of treatment effect is needed, the dose of Dysport can be increased to 80 units per eye. The maximum dose administered should not exceed a total dose of 120 units per eye.
In the treatment of upper limb spasticity in adults:Depending on the doctor's decision, Dysport will be injected at a dose of 500 to 1000 units. Your doctor may divide this dose, injecting it into individual muscles of the arm and shoulder. Relief of muscle spasms should usually occur within a week. Injections will be performed approximately every 12-16 weeks.
In the treatment of lower limb spasticity in adults:Dysport is usually administered at a dose of 1500 units. Your doctor may divide this dose, injecting it into individual muscles of the lower limb. Injections will be performed approximately every 12-16 weeks.
In the treatment of upper and lower limb spasticity in adults:If there is a need to administer the medicine to both the upper and lower limbs during one therapeutic session, your doctor may divide the dose of Dysport between the muscles of these two parts of the body. In this case, the total dose of the medicine should not exceed 1500 units.
In the treatment of excessive axillary sweating:Dysport is usually administered at a dose of 100 units per axilla. Your doctor will divide this dose, injecting it into 10 places in the axilla. The maximum effect should be visible about 2 weeks after administration. The maximum dose administered should not exceed 200 units per axilla.
Using a higher dose of Dysport than recommended
In the event of administration of a higher dose of Dysport than recommended, the patient may experience muscle weakness other than those into which the medicine was injected. Symptoms of overdose may not occur immediately after injection. If they occur, you should contact your doctor immediately. If you experience difficulty breathing, swallowing, or speaking, you should go to the nearest medical facility.
4. Possible side effects
Like all medicines, Dysport can cause side effects, although not everybody gets them.
You should immediately inform your doctor if:
- you experience problems with swallowing, breathing, or speech
- you experience difficulty breathing with or without swelling of the face, lips, tongue, and/or throat, redness of the skin, or an itchy, lumpy rash (hives). The occurrence of such symptoms may indicate that you are allergic to Dysport. The likelihood of side effects is classified as follows:
| Frequency of occurrence | |
| Very common | may occur in more than 1 in 10 people |
| Common | may occur in up to 1 in 10 people |
| Uncommon | may occur in up to 1 in 100 people |
| Rare | may occur in up to 1 in 1000 people |
| Frequency not known | frequency cannot be estimated from the available data |
Some side effects may occur in all patients treated with Dysport, while others may depend on the type of symptoms being treated. You should read the information related to the specific symptom.
The following side effects have been reported (regardless of indication):
Common:
- bruising and/or pain at the injection site
- general weakness
- fatigue
- flu-like symptoms
- Uncommon:
- itching
- Rare:
- rash, neuralgia with muscle atrophy
- Frequency not known (frequency cannot be estimated from the available data):
- numbness
- muscle atrophy
- Other side effects have also been reported related to the spread of the medicine beyond the injection site (increased muscle weakness, swallowing difficulties, or choking with a fatal outcome in very rare cases).
Symptomatic treatment of focal spasticity of the lower limbs in children with cerebral palsy
The following side effects have been reported:
- Common:
- muscle pain
- weakness of lower limb muscle strength
- urinary incontinence
- flu-like symptoms
- pain, redness, bruising at the injection site
- gait disturbance
- fatigue
- falls
- Uncommon:
- decrease in strength and weakness
Symptomatic treatment of focal spasticity of the upper limbs in children with cerebral palsy
The following side effects have been reported:
- Common:
- muscle weakness
- muscle pain
- flu-like symptoms
- fatigue
- itching of the skin, bruising, pain, swelling, and rash at the injection site
- rash
- Uncommon:
- decrease in strength and weakness
Simultaneous treatment of focal spasticity of the upper and lower limbs in children with cerebral palsy
No additional side effects have been reported during simultaneous treatment of the upper and lower limbs during the same therapeutic session compared to separate administration to the upper or lower limb.
Treatment of urinary incontinence due to uncontrolled muscle contractions of the urinary bladder
The following side effects have been reported:
- Common:
- hematuria*
- constipation
- bacteria in the urine*
- erectile dysfunction, sometimes referred to as impotence
- urinary tract infection*
- headache
- fever
- Uncommon:
- numbness
- muscle weakness
- urinary bladder pain*
- uncontrolled reflex reaction of the body (autonomic dysreflexia)*
- inability to empty the urinary bladder (urinary retention)
- bleeding from the urinary bladder or from the tube that carries urine out of the body (urethra)
Treatment of cervical dystonia
The following side effects have been reported:
- Very common:
- swallowing difficulties (dysphagia)
- dry mouth
- muscle weakness
- Common:
- headache
- facial muscle weakness
- dizziness
- blurred vision
- decreased visual acuity
- shortness of breath
- neck pain
- musculoskeletal pain
- muscle pain
- pain in the arms and fingers
- muscle stiffness
- change in voice (dysphonia)
- Uncommon:
- double vision
- drooping of the lower or upper eyelid
- muscle atrophy
- weakness of the jaw muscles
- nausea
- Rare:
- aspiration (choking)
Treatment of blepharospasm and hemifacial spasm
The following side effects have been reported:
- Very common:
- drooping of the lower or upper eyelid
- Common:
- dry eyes
- double vision
- increased tearing
- eyelid swelling
- facial weakness
- Uncommon:
- facial nerve palsy
- Rare:
- eye muscle palsy (limited ability to move the eyeball)
- eyelid ptosis
Treatment of upper limb spasticity in adults
The following side effects have been reported:
- Common:
- muscle weakness
- musculoskeletal pain
- pain in the hands and fingers
- pain, redness, swelling at the injection site
- fatigue, weakness
- flu-like symptoms
- Uncommon:
- swallowing difficulties
Treatment of lower limb spasticity in adults
The following side effects have been reported:
- Common:
- falls
- muscle weakness
- muscle pain
- swallowing difficulties (dysphagia)
- weakness
- fatigue
- flu-like symptoms
- bruising, pain, rash, itching of the skin at the injection site
Treatment of excessive axillary sweating
The following side effects have been reported:
- Common:
- shortness of breath
- compensatory sweating (increased sweating in areas other than the armpits)
- back pain, shoulder and neck pain
- muscle pain in the back and calves
- Uncommon:
- dizziness
- headache
- tingling or numbness of the hands or feet
- involuntary twitching of the eyelids
- flushing of the face
- nasal bleeding
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist.
5. How to store Dysport
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging.
Dysport should be stored in a refrigerator (2°C - 8°C). Do not freeze.
The solution should be used immediately after reconstitution. After reconstitution, the solution can be stored for up to 24 hours in a refrigerator (2°C – 8°C).
Expiry date (EXP)
Batch number (Lot)
6. Package contents and other information
What Dysport contains
- The active substance of Dysport is botulinum toxin type A, 300 or 500 units/vial
- The other ingredients are human albumin and lactose monohydrate. Clostridium botulinum type A neurotoxin complex
What Dysport looks like and contents of the pack
Dysport is a white powder for solution for injection. The packs contain 1 or 2 vials.
Marketing authorization holder
Ipsen Pharma, 65 Quai Georges Gorse, 92100 Boulogne-Billancourt, France
Importer
Ipsen Manufacturing Ireland Limited, Blanchardstown Industrial Park
Blanchardstown, Dublin 15, Ireland
Date of last revision of the leaflet:June 2022
Additional information or a leaflet in a format suitable for the blind or visually impaired can be obtained by contacting:
Ipsen Poland Sp. z o.o.
ul. Chmielna 73
00-801 Warsaw
tel.: 22 653 68 00
fax: 22 653 68 22
Website: https://smz.ezdrowie.gov.pl
The marketing authorization holder's logo
Information intended for healthcare professionals only:
In order to improve the identifiability of biological medicinal products, the name and batch number of the administered product should be clearly recorded.
The units of Dysport are specific to this product and are not interchangeable with units of other botulinum toxin products.
Dysport should only be administered by appropriately trained physicians.
The uncoated rubber stopper should be wiped with alcohol immediately before puncture with a needle. Sterile needles of size 23 or 25 should be used.
The following instructions describe the preparation of Dysport for injection. The volumes of diluent shown allow for the preparation of concentrations suitable for use in the specific indication, with the exception of the indication for urinary incontinence due to neurogenic overactivity of the detrusor muscle, for which there are specific instructions (see below).
* 0.9% sodium chloride solution for injection without preservatives
Focal spasticity in children with cerebral palsy aged 2 years or older
Focal spasticity of the lower limbs in children with cerebral palsy
The maximum total dose of Dysport administered during one therapeutic session should not exceed 15 units/kg body weight if injected into one lower limb or 30 units/kg body weight if injected into both lower limbs. The total dose of Dysport administered during one therapeutic session should not exceed 1000 units or 30 units/kg body weight, whichever is smaller. The total dose of Dysport should be divided between the muscles of the lower limbs affected by spasticity. If possible, the dose per muscle should be divided into more than one injection site. The maximum volume of Dysport injected into one site should not exceed 0.5 ml. The recommended dosing is shown in the table below.
| Concentration in units per ml | Volume of diluent* added to the 300 unit vial | Volume of diluent* added to the 500 unit vial |
| 500 units 200 units 100 units | 0.6 ml 1.5 ml 3 ml | 1 ml 2.5 ml 5 ml |
| Muscle | Recommended dose range per muscle per lower limb (units/kg body weight) | Number of injection sites per muscle |
| Distal | ||
| Gastrocnemius muscle | 5 to 15 units/kg | Up to 4 |
| Soleus muscle | 4 to 6 units/kg | Up to 2 |
| Posterior tibial muscle | 3 to 5 units/kg | Up to 2 |
| Proximal | ||
| Hamstring muscles | 5 to 6 units/kg | Up to 2 |
| Hip adductor muscles | 3 to 10 units/kg | Up to 2 |
| Total dose per lower limb | Up to 15 units/kg/limb if only the distal muscles are injected, only the proximal muscles are injected, or if both are injected. | |
Focal spasticity of the upper limbs in children with cerebral palsy aged 2 years or older
The maximum dose of Dysport administered during one therapeutic session for unilateral injection should not exceed 16 units/kg body weight or 640 units, whichever is smaller. For bilateral injection, the maximum dose of Dysport administered during one therapeutic session should not exceed 21 units/kg body weight or 840 units, whichever is smaller.
The total dose administered should be divided between the muscles of the upper limb(s) affected by spasticity. No more than 0.5 ml of Dysport should be injected into one site. The recommended dosing is shown in the table below.
Dosing of Dysport by muscle in children with upper limb spasticity
| Muscle | Recommended dose range per muscle per upper limb (units/kg body weight) | Number of injection sites per muscle |
| Biceps brachii muscle | 3 to 6 units/kg | Up to 2 |
| Brachialis muscle | 1.5 to 3 units/kg | 1 |
| Brachioradialis muscle | 3 to 6 units/kg | Up to 2 |
| Pronator teres muscle | 1 to 2 units/kg | 1 |
| Pronator quadratus muscle | 0.5 to 1 unit/kg | 1 |
| Flexor carpi radialis muscle | 2 to 4 units/kg | Up to 2 |
| Flexor carpi ulnaris muscle | 1.5 to 3 units/kg | 1 |
| Flexor digitorum profundus muscle | 1 to 2 units/kg | 1 |
| Flexor digitorum superficialis muscle | 1.5 to 3 units/kg | Up to 4 |
| Thenar muscles | 0.5 to 1 unit/kg | 1 |
| Opponens pollicis muscle | 0.5 to 1 unit/kg | 1 |
| Total dose | Up to 16 units/kg/upper limb (not exceeding 21 units/kg in the case of injection into both upper limbs) | |
Focal spasticity of the upper and lower limbs in children with cerebral palsy aged 2 years or older
Dosing
In the case of simultaneous treatment of upper and lower limb spasticity in children, you should refer to the section on dosing for the specific indication, i.e. treatment of focal spasticity of the upper or lower limbs in children. In the case of simultaneous treatment, the dose of Dysport should not exceed the total dose administered during one therapeutic session of 30 units/kg body weight or 1000 units, whichever is smaller.
Re-treatment of the upper and lower limbs should be considered when the effect of the previous injection has decreased, but no earlier than 12-16 weeks after the previous therapeutic session. The optimal time for re-treatment should be determined based on the progression of the disease and the response to treatment.
Administration
In the treatment of spasticity of the lower limbs or upper limbs associated with cerebral palsy or both indications, Dysport solution is used, which is prepared by reconstituting the product in 0.9% (9 mg/ml) sodium chloride solution for injection.
Urinary incontinence due to neurogenic overactivity of the detrusor muscle
Dosing
The recommended dose is 600 units. In the case of an inadequate response to treatment or in patients with a severe form of the disease (e.g. depending on the severity of objective and subjective symptoms and/or urodynamic parameters), a dose of 800 units may be used.
Dysport should be administered to patients who regularly undergo clean intermittent catheterization.
The total dose should be divided into 30 injections into the detrusor muscle, evenly distributed throughout the muscle, avoiding the trigone of the bladder. Dysport is injected using a flexible or rigid cystoscope, with each injection performed at a depth of approximately 2 mm, administering 0.5 ml to each site. To ensure that the entire dose is administered, the last injection should include approximately 0.5 ml of sterile sodium chloride solution.
Administration
Dysport is administered by injection into the detrusor muscle, as described above. In the treatment of urinary incontinence due to neurogenic overactivity of the detrusor muscle, Dysport solution containing 600 or 800 units in 15 ml is used, which is prepared by reconstituting the product in 0.9% (9 mg/ml) sodium chloride solution for injection.
Instructions for preparing the solution for the indication of urinary incontinence due to neurogenic overactivity of the detrusor muscle:
The goal is to achieve the required volume of 15 ml of Dysport solution for injection after reconstitution, evenly divided into two 10 ml syringes, each containing 7.5 ml of the solution at the same concentration.
After reconstitution, the product should be used immediately.
Instructions for preparing the solution using 500 unit vials
- For a dose of 600 units:each of the 2 vials containing 500 units should be reconstituted using 2.5 ml of 9 mg/ml sodium chloride solution for injection without preservatives per vial. 1.5 ml should be drawn from the first vial into the first 10 ml syringe and 1.5 ml from the second vial into the second 10 ml syringe. The reconstitution should be completed by drawing 6 ml of 9 mg/ml sodium chloride solution without preservatives into each syringe and gently mixing. This will result in a total of 600 units of Dysport solution in two 10 ml syringes, each containing 7.5 ml of the solution.
- For a dose of 800 units:each of the 2 vials containing 500 units should be reconstituted using 2.5 ml of 9 mg/ml sodium chloride solution for injection without preservatives per vial. 2 ml should be drawn from the first vial into the first 10 ml syringe and 2 ml from the second vial into the second 10 ml syringe. The reconstitution should be completed by drawing 5.5 ml of 9 mg/ml sodium chloride solution without preservatives into each syringe and gently mixing. This will result in a total of 800 units of Dysport solution in two 10 ml syringes, each containing 7.5 ml of the solution.
Instructions for preparing the solution using 300 unit vials
- For a dose of 600 units:each of the 2 vials containing 300 units should be reconstituted using 1.5 ml of 9 mg/ml sodium chloride solution for injection without preservatives per vial. The entire 1.5 ml should be drawn from the first vial into the first 10 ml syringe and the entire 1.5 ml from the second vial into the second 10 ml syringe. The reconstitution should be completed by drawing 6 ml of 9 mg/ml sodium chloride solution without preservatives into each syringe and gently mixing. This will result in a total of 600 units of Dysport solution in two 10 ml syringes, each containing 7.5 ml of the solution.
- For a dose of 800 units:each of the 3 vials containing 300 units should be reconstituted using 1.5 ml of 9 mg/ml sodium chloride solution for injection without preservatives per vial. 1.5 ml should be drawn from the first vial into the first 10 ml syringe and 0.5 ml from the second vial into the first 10 ml syringe. 0.5 ml should be drawn from the second vial into the second 10 ml syringe and the entire 1.5 ml from the third vial into the second 10 ml syringe. The reconstitution should be completed by drawing 5.5 ml of 9 mg/ml sodium chloride solution without preservatives into each syringe and gently mixing. This will result in a total of 800 units of Dysport solution in two 10 ml syringes, each containing 7.5 ml of the solution.
Instructions for preparing the solution using a combination of 500 unit and 300 unit vials (only for a dose of 800 units)
- For a dose of 800 units:the vial containing 500 units should be reconstituted using 2.5 ml of 9 mg/ml sodium chloride solution for injection without preservatives; the vial containing 300 units should be reconstituted using 1.5 ml of 9 mg/ml sodium chloride solution for injection without preservatives. 2 ml should be drawn from the vial containing 500 units into the first 10 ml syringe. 0.5 ml should be drawn from the vial containing 500 units and the entire 1.5 ml from the vial containing 300 units into the second 10 ml syringe. The reconstitution should be completed by drawing 5.5 ml of 9 mg/ml sodium chloride solution without preservatives into each syringe and gently mixing. This will result in a total of 800 units of Dysport solution in two 10 ml syringes, each containing 7.5 ml of the solution.
Cervical dystonia
Dosing
The recommended doses for the treatment of cervical dystonia apply to adults of any age, with a normal body weight, in whom no muscle wasting of the neck muscles is observed. In individuals with reduced neck muscle mass, e.g. due to significant underweight or elderly individuals, the dose may be reduced.
The initial recommended dose for the treatment of cervical dystonia is 500 units, administered in divided doses into two or three of the most active neck muscles.
For continued treatment, doses may be adjusted according to the treatment effect and observed side effects. It is recommended to administer 250 to 1000 units, although the use of higher doses within this range may be associated with an increased frequency and severity of side effects, particularly difficulty swallowing. The maximum administered dose should not exceed 1000 units. Relief of cervical dystonia symptoms should usually occur within a week of the first injection. Injections should be repeated approximately every 16 weeks or as needed to maintain treatment, but no more frequently than every 12 weeks.
In the case of cervical dystonia with rotation, a total dose of 500 units should be used, administering 350 units to the sternocleidomastoid muscle on the side towards which the chin is turned/head is tilted, and 150 units to the trapezius muscle on the opposite side to the rotation.
In the case of cervical dystonia with lateral tilt of the head, a total dose of 500 units is recommended, administering 350 units to the sternocleidomastoid muscle on the side towards which the head is tilted, and 150 units to the trapezius muscle on the same side.
In cases where there is also elevation of the shoulders, treatment of the rhomboid and levator scapulae muscles may also be required, if significant hypertrophy of these muscles is observed in the EMG examination. When injecting into three muscles, 500 units of Dysport should be divided as follows: 300 units to the sternocleidomastoid muscle, 100 units to the trapezius muscle, and 100 units to the third muscle.
In the case of cervical dystonia with retrocollis, a total dose of 500 units should be administered, injecting 250 units into each of the sternocleidomastoid muscles. Injections into both sternocleidomastoid muscles may increase the risk of neck muscle weakness.
In all other forms of cervical dystonia, identification and treatment of the most active muscles depend on the specialist knowledge of physicians and the results of the EMG examination. EMG should be used in the diagnosis of all complex forms of cervical dystonia, for re-evaluation after ineffective injections in non-complicated cases, and to guide injections into deeply located muscles or in patients with obesity, in whom palpation of the neck muscles is difficult.
Administration
In the treatment of cervical dystonia, a solution containing 500 units in 1 ml is used, which is prepared by reconstituting Dysport 300 units in 0.6 ml or Dysport 500 units in 1 ml of 0.9% (w/v) sodium chloride solution for injection. Dysport is administered intramuscularly in the manner described above.
Blepharospasm and Hemifacial Spasm
Dosage
Administer 10 units (0.05 ml) of the medicinal product medially and 10 units (0.05 ml) laterally with respect to the junction of the eyelid and orbital parts of both the upper (3 and 4) and lower (5 and 6) parts of the orbicularis oculi muscle of each eye.
To minimize the risk of eyelid ptosis, injections should be avoided near the levator palpebrae superioris.
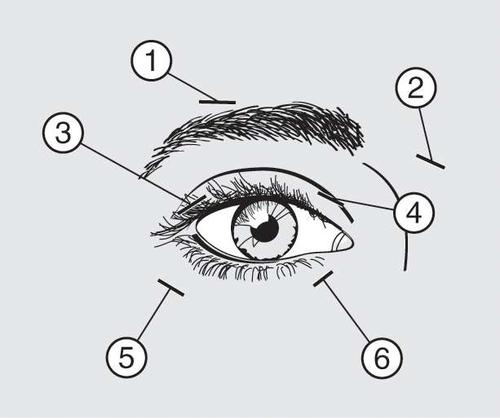
When injecting the product into the upper eyelid, the needle should be withdrawn from the center of the eyelid to avoid administering the product into the levator palpebrae superioris muscle. The scheme above is intended to help with the proper distribution of the product administration. The onset of relief from symptoms can be expected within two to four days after injection, but the maximum effect usually occurs after two weeks.
Injections should be repeated approximately every 12 weeks or as frequently as necessary to prevent the recurrence of symptoms, but no more frequently than every 12 weeks.
If the response to initial treatment is deemed insufficient, it may be necessary to increase the dose to 60 units during subsequent visits in the following manner: 10 units (0.05 ml) medially and 20 units (0.1 ml) laterally, 80 units: 20 units (0.1 ml) medially and 20 units (0.1 ml) laterally, or up to 120 units: 20 units (0.1 ml) medially and 40 units (0.2 ml) laterally above and below each eye, using the injection technique described above. Additionally, Dysport can be injected into the procerus muscle above the eyebrows (1 and 2) if spasms in this area cause visual disturbances.
In cases of unilateral blepharospasm, injections should be limited to the affected eye. Patients with hemifacial spasm should be treated in the same manner as those with unilateral blepharospasm. The recommended doses are applicable to adults of all ages, including the elderly.
Children: The efficacy and safety of this medicinal product for the treatment of blepharospasm and hemifacial spasm in children have not been established.
Method of administration
In the treatment of blepharospasm and hemifacial spasm, a solution containing 200 units in 1 ml is used, which is obtained after reconstitution of the Dysport 300 units in 1.5 ml or Dysport 500 units in 2.5 ml 0.9% (w/v) sodium chloride injection solution. Dysport is administered in subcutaneous injections medially and laterally with respect to the junction of the eyelid and orbital parts of both the upper and lower parts of the orbicularis oculi muscle of each eye.
Focal Spasticity of Upper and Lower Limbs in Adults
Upper Limb
Dosage
In clinical trials, doses of 500 and 1000 units were divided among selected muscles in treatment sessions according to the scheme presented below.
No more than 1 ml of solution should be injected into one site.
Lower Limb
Dosage
The total dose of the medicinal product should not exceed 1500 units. No more than 1 ml of solution should be injected into one site.
| Muscles injected | Recommended dose of Dysport (units) |
| Flexor carpi radialis (FCR) muscle Flexor carpi ulnaris (FCU) muscle | 100-200 units 100-200 units |
| Flexor digitorum profundus (FDP) muscle Flexor digitorum superficialis (FDS) muscle Flexor pollicis longus muscle Flexor pollicis brevis muscle | 100-200 units 100-200 units 100-200 units 25-50 units |
| Biceps brachii muscle Brachioradialis muscle Biceps brachii (BB) muscle Pronator teres muscle | 200-400 units 100-200 units 200-400 units 100-200 units |
| Triceps brachii (long head) muscle Pectoralis major muscle Subscapularis muscle Latissimus dorsi muscle | 150-300 units 150-300 units 150-300 units 150-300 units |
| Muscles injected | Recommended dose of Dysport (units) | Number of injections per muscle |
| Distal | ||
| Pectoralis muscle | 300-550 units | 2-4 |
| Gastrocnemius muscle | ||
| Medial head | 100-450 units | 1-3 |
| Lateral head | 100-450 units | 1-3 |
| Tibialis posterior muscle | 100-250 units | 1-3 |
| Flexor digitorum longus muscle | 50-200 units | 1-2 |
| Flexor digitorum brevis muscle | 50-200 units | 1-2 |
| Flexor hallucis longus muscle | 50-200 units | 1-2 |
| Flexor hallucis brevis muscle | 50-100 units | 1-2 |
| Proximal | ||
| Rectus femoris muscle | 100-400 units | 1-3 |
| Hamstring muscles | 100-400 units | 1-3 |
| Adductor magnus muscle | 100-300 units | 1-3 |
| Adductor longus muscle | 50-150 units | 1-2 |
| Adductor brevis muscle | 50-150 units | 1-2 |
| Gracilis muscle | 100-200 units | 1-3 |
| Gluteus maximus muscle | 100-400 units | 1-2 |
The degree and form of muscle spasticity at the time of repeat injection may require a change in the dose of Dysport and the injection site.
Upper and Lower Limb
If during one therapeutic session there is a need to administer the product to both the upper and lower limb muscles, the dose of Dysport should be adjusted according to the patient's needs, remembering that the total dose should not exceed 1500 units.
Method of administration
In the treatment of focal spasticity of upper and lower limbs, a solution containing 100, 200, or 500 units in 1 ml is used, which is obtained after reconstitution of Dysport in 0.9% (w/v) sodium chloride injection solution. Dysport is administered in intramuscular injections into the specified muscles above.
Axillary Hyperhidrosis
Dosage
The recommended initial dose for the treatment of axillary hyperhidrosis is 100 units per axilla. If the desired effect is not achieved, the dose can be increased to 200 units per axilla in the next administration. The maximum administered dose should not exceed 200 units per axilla.
The area to be injected must be previously tested with the iodine-starch test. Both axillae must be thoroughly cleaned and disinfected. Intra-dermal injections should be performed at ten sites. 10 units should be administered at each site, i.e., 100 units per axilla. The maximum effect should be visible approximately two weeks after the injection.
Method of administration
In the treatment of axillary hyperhidrosis, a solution containing 200 units in 1 ml is used, which is obtained after reconstitution of the Dysport 300 units in 1.5 ml or Dysport 500 units in 2.5 ml 0.9% (w/v) sodium chloride injection solution. Dysport is administered in intra-dermal injections as described above.
Detailed information on dosing and administration can be found in the Summary of Product Characteristics.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterIpsen Manufacturing Ireland Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DisportDosage form: Solution, 200 U Speywood/mlActive substance: botulinum toxinPrescription requiredDosage form: Powder, 125 Speywood unitsActive substance: botulinum toxinPrescription requiredDosage form: Powder, 125 Speywood unitsActive substance: botulinum toxinPrescription required
Alternatives to Disport in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Disport in Ukraine
Alternative to Disport in Spain
Online doctors for Disport
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Disport – subject to medical assessment and local rules.














