

Donepex

Ask a doctor about a prescription for Donepex

How to use Donepex
Leaflet accompanying the packaging: patient information
Donepex, 5 mg, coated tablets
Donepex, 10 mg, coated tablets
Donepezilhydrochloride
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Donepex and what is it used for
- 2. Important information before taking Donepex
- 3. How to take Donepex
- 4. Possible side effects
- 5. How to store Donepex
- 6. Contents of the pack and other information
1. What is Donepex and what is it used for
Donepex contains the active substance donepezil hydrochloride, which is a reversible inhibitor of acetylcholinesterase, the main enzyme that breaks down acetylcholine in the brain. Donepex is indicated for the symptomatic treatment of mild to moderate Alzheimer's disease.
2. Important information before taking Donepex
When not to take Donepex
- If the patient is allergic to the active substance, piperidine derivatives, or any of the other ingredients of this medicine (listed in section 6).
- If the patient is pregnant.
Warnings and precautions
Particular caution should be exercised:
- in patients with heart disease: sick sinus syndrome or conduction disorders (sinoatrial block, atrioventricular block);
- in patients with urinary tract disorders (with difficulty urinating);
- in patients with stomach or duodenal ulcers;
- in patients with seizure disorders;
- in patients with asthma or other chronic lung diseases;
- in patients with liver disease or a history of hepatitis;
- in patients who are to undergo surgery or dental procedures. The patient should inform the doctor that they are taking Donepex;
- in patients taking antipsychotic drugs. The patient should consult a doctor, even if the above warnings refer to situations that have occurred in the past.
Before starting to take Donepex, the patient should discuss it with their doctor or pharmacist if they:
- have or have had heart disorders (such as irregular or very slow heartbeat, heart failure, myocardial infarction);
- have or have had a heart disorder called "prolonged QT interval" or have previously been diagnosed with certain heart rhythm disorders called torsades de pointes or have a family history of "prolonged QT interval";
- have or have had low magnesium or potassium levels in the blood.
Donepex with other medicines
The patient should tell their doctor or pharmacist about all medicines they are taking or have recently taken, as well as any medicines they plan to take. It is particularly important to inform the doctor about taking any of the following medicines:
- medicines used to treat heart rhythm disorders, such as amiodarone, sotalol;
- medicines used to treat depression, such as citalopram, escitalopram, amitriptyline, fluoxetine;
- antipsychotic drugs, such as pimozide, sertindole, ziprasidone;
- medicines used to treat bacterial infections, such as clarithromycin, erythromycin, levofloxacin, moxifloxacin, rifampicin;
- antifungal drugs, such as ketoconazole, itraconazole;
- other medicines used to treat Alzheimer's disease, such as galantamine;
- painkillers or medicines used to treat arthritis, such as acetylsalicylic acid, non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or diclofenac sodium;
- anticholinergic drugs, such as tolterodine;
- antiepileptic drugs, such as phenytoin, carbamazepine;
- medicines used to treat heart diseases, such as quinidine, beta-blockers (propranolol and atenolol);
- muscle relaxants, such as diazepam, succinylcholine;
- general anesthetics;
- over-the-counter medicines, such as herbal preparations.
A patient who is to undergo anesthesia should inform their doctor that they are taking Donepex.
Donepex with food, drink, and alcohol
Food does not affect the absorption of the medicine. The patient should not consume alcohol while taking Donepex.
Pregnancy and breastfeeding
In pregnancy, during breastfeeding, or if the patient suspects they are pregnant, or plans to become pregnant, they should consult a doctor or pharmacist before taking this medicine. The use of Donepex during pregnancy is contraindicated. Women taking Donepex should not breastfeed.
Driving and using machines
Donepex has a minor or moderate effect on the ability to drive or operate machines. Alzheimer's disease may impair the ability to drive or operate machines. Additionally, Donepex may cause fatigue, dizziness, and muscle cramps, especially at the beginning of treatment or after increasing the dose. The doctor will assess the patient's ability to continue driving or operating machines while taking Donepex.
Donepex contains lactose monohydrate
If the patient has been diagnosed with intolerance to some sugars, they should consult their doctor before taking the medicine.
3. How to take Donepex
This medicine should always be taken according to the doctor's instructions. In case of doubts, the patient should consult their doctor or pharmacist.
Use in adults, including elderly patients
The initial dose is 5 mg per day (taken once a day). The patient should continue taking 5 mg per day for at least one month, which will allow the doctor to assess the effectiveness of the treatment. After a clinical assessment of the therapy for one month at a dose of 5 mg per day, the doctor may increase the dose of the medicine to 10 mg per day (taken once a day). The maximum recommended daily dose is 10 mg. The tablets should be taken orally, in the evening, just before bedtime. If the patient experiences unusual dreams, nightmares, or difficulty falling asleep (see section 4), the doctor may recommend taking Donepex in the morning. If the patient feels that the effect of Donepex is too strong or too weak, they should consult their doctor.
Use in children
The use of Donepex in children is not recommended.
Use in patients with renal impairment
Patients with impaired renal function may use a similar dosing schedule as patients with normal renal function.
Use in patients with hepatic impairment
In patients with mild and moderate hepatic impairment, the dose should be increased according to individual patient tolerance. There are no data on the use of the medicine in patients with severe hepatic impairment.
Overdose of Donepex
In case of overdose, the patient should immediately inform their doctor or go to the nearest hospital. Symptoms suggesting overdose may include: severe nausea, vomiting, salivation, excessive sweating, bradycardia (slow heart rate), hypotension, respiratory depression, collapse, and seizures. Progressive muscle weakness leading to death may also occur in the case of respiratory muscle weakness. Depending on the symptoms, the doctor will provide appropriate treatment.
Missed dose of Donepex
The patient should take the missed dose as soon as possible, unless it is close to the time of the next dose. In this case, they should take the next dose at the scheduled time. The patient should not take a double dose to make up for the missed dose.
4. Possible side effects
Like all medicines, Donepex can cause side effects, although not everybody gets them.
Severe side effects:
If any of the following severe side effects occur, the patient should immediately consult a doctor, as they may require urgent medical attention:
- liver dysfunction, including hepatitis. Symptoms of hepatitis include nausea or vomiting, loss of appetite, general malaise, fever, itching, yellowing of the skin and eyes, dark urine (Frequency: rare, may occur in less than 1 in 1,000 patients);
- stomach or duodenal ulcers. Symptoms of ulcers include stomach pain and discomfort in the upper abdomen (Frequency: uncommon, may occur in less than 1 in 100 patients);
- gastrointestinal bleeding. It may cause black, tarry stools or blood in the stool (Frequency: uncommon, may occur in less than 1 in 100 patients);
- seizures or convulsions (Frequency: uncommon, may occur in less than 1 in 100 patients);
- fever with muscle stiffness, sweating, or decreased consciousness (a condition called neuroleptic malignant syndrome) (Frequency: very rare, may occur in less than 1 in 10,000 patients);
- weakness, tenderness, or muscle pain, especially if accompanied by malaise, high fever, or dark urine. These symptoms may be caused by abnormal muscle cell breakdown, which can be life-threatening and lead to kidney dysfunction (a condition called rhabdomyolysis) (Frequency: very rare, may occur in less than 1 in 10,000 patients);
- rapid, irregular heartbeat, fainting, which may be symptoms of a life-threatening condition called torsades de pointes (Frequency: unknown, cannot be estimated from available data).
Other side effects include:
- Very common side effects (may occur in more than 1 in 10 patients):
- Diarrhea,
- Nausea
- Headache
Common side effects (may occur in less than 1 in 10 patients):
- Cold
- Anorexia
- Hallucinations, unusual dreams, including nightmares, agitation, aggressive behavior
- Fainting, dizziness, insomnia
- Vomiting, gastrointestinal disorders
- Rash, itching
- Muscle cramps
- Urinary incontinence
- Fatigue, pain
- Accidents
Uncommon side effects (may occur in less than 1 in 100 patients):
- Bradycardia (slow heart rate)
- Slightly increased muscle creatine kinase activity in the blood
Rarely reported side effects (may occur in less than 1 in 1,000 patients):
- Extrapyramidal symptoms (movement disorders affecting the nervous system, such as: muscle stiffness, reduced facial expressions, slowed movement, restlessness, involuntary muscle contractions, and involuntary movements)
- Sinoatrial block, atrioventricular block
Unknown frequency (cannot be estimated from available data)
- Changes in heart function visible on an ECG called "prolonged QT interval"
- Increased libido, hypersexuality
- A symptom called "Pisa tower syndrome" (involving involuntary muscle contraction with abnormal twisting of the body and head to one side).
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Donepex
The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month. Store in the original packaging.
Do not use this medicine if the packaging is damaged or shows signs of tampering.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Donepex contains
- The active substance of the medicine is donepezil hydrochloride. Each Donepex 5 mg coated tablet contains 5 mg of donepezil hydrochloride.
- Other ingredients are:
core: microcrystalline cellulose, lactose monohydrate, cornstarch, hypromellose, magnesium stearate, coating: Opadry HP White (macrogol, titanium dioxide (E171), talc, polyvinyl alcohol), yellow lake quinoline (E104). Each Donepex 10 mg coated tablet contains 10 mg of donepezil hydrochloride.
- Other ingredients are: core: microcrystalline cellulose, lactose monohydrate, cornstarch, hypromellose, magnesium stearate, coating: Opadry HP White (macrogol, titanium dioxide (E171), talc, polyvinyl alcohol).
What Donepex looks like and contents of the pack
Donepex 5 mg is a yellow, round, biconvex coated tablet. Donepex 10 mg is a white, round, biconvex coated tablet. Available packs: 28 tablets of 5 mg or 10 mg in a polyethylene container with a polypropylene screw cap and a desiccant in the container or PVC/PVDC/Aluminum blisters in a cardboard box.
Marketing authorization holder and manufacturer:
Marketing authorization holder:
Celon Pharma S.A. ul. Ogrodowa 2A 05-092 Łomianki/Kiełpin tel.: (22) 75-15-933, e-mail: [email protected]
Manufacturer:
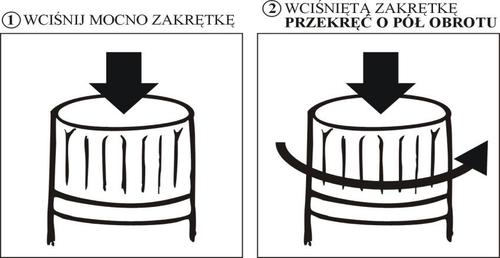
Celon Pharma S.A. ul. Marymoncka 15 05-152 Kazuń Nowy For more detailed information about this medicine, the patient should contact the marketing authorization holder: Celon Pharma S.A. ul. Ogrodowa 2A, Kiełpin 05-092 Łomianki tel.: (22) 75-15-933, e-mail: [email protected] To protect the medicine from unauthorized access and children, the packaging cap is equipped with a tamper-evident ring and a child-resistant opening mechanism. The patient should follow the instructions below to open the packaging correctly: PRESS THE CAP FIRMLY TURN THE PRESSED CAP HALF A TURN
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterCelon Pharma S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DonepexDosage form: Tablets, 5 mgActive substance: donepezilManufacturer: Apotex Nederland B.V. SPECIAL PRODUCTS LINE S.p.A.Prescription requiredDosage form: Tablets, 10 mgActive substance: donepezilManufacturer: Apotex Nederland B.V. SPECIAL PRODUCTS LINE S.p.A.Prescription requiredDosage form: Tablets, 5 mgActive substance: donepezilManufacturer: Fareva AmboisePrescription required
Alternatives to Donepex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Donepex in Spain
Alternative to Donepex in Ukraine
Online doctors for Donepex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Donepex – subject to medical assessment and local rules.











