
How to use Dalacin
Leaflet attached to the packaging: patient information
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
DALACIN
20 mg/g (2%), vaginal cream
Clindamycin
It is necessary to carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- The leaflet should be kept so that it can be re-read if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for one person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Dalacin and what is it used for
- 2. Important information before using Dalacin
- 3. How to use Dalacin
- 4. Possible side effects
- 5. How to store Dalacin
- 6. Package contents and other information
1. What is Dalacin and what is it used for
Dalacin vaginal cream contains clindamycin - an antibiotic with antibacterial action.
Dalacin vaginal cream is used to treat bacterial vaginosis caused by susceptible microorganisms.
2. Important information before using Dalacin
When not to use Dalacin
- If the patient is allergic to clindamycin, lincomycin, or any of the other ingredients of this medicine (listed in section 6).
- If the patient has had colitis after using antibiotics.
Warnings and precautions
Before starting to use Dalacin, the patient should discuss it with their doctor.
Using Dalacin vaginal cream may cause the growth of non-susceptible microorganisms in the vagina, especially yeast.
Clindamycin, like other antibiotics, is associated with the risk of diarrhea and pseudomembranous colitis, even though the antibiotic is absorbed from the cream only to a minimal extent. The patient should consult their doctor if they experience diarrhea, especially severe and persistent, during or after treatment. This may be a sign of pseudomembranous colitis (usually caused by Clostridium difficile), a complication of antibiotic therapy. The patient should not take medications that suppress intestinal peristalsis or have a constipating effect.
It is recommended that during the use of Dalacin vaginal cream, the patient should not have sexual intercourse or use vaginal tampons or douching.
Dalacin vaginal cream contains ingredients that may damage rubber and latex products, such as vaginal diaphragms or condoms, reducing their effectiveness in preventing pregnancy or protecting against sexually transmitted diseases, including AIDS. Therefore, the use of condoms or vaginal diaphragms is not recommended during treatment.
Children and adolescents
The safety and efficacy of Dalacin vaginal cream in children have not been established.
Dalacin and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. Dalacin vaginal cream may interact with other medicines used at the same time.
If the patient is to undergo surgery, they should inform their doctor about the use of Dalacin vaginal cream. Clindamycin may enhance the effect of muscle relaxants used during surgery.
If the patient is taking erythromycin (an antibiotic), they should inform their doctor before starting to use Dalacin vaginal cream.
Due to the lack of data, it is recommended that Dalacin vaginal cream should not be used concurrently with other vaginal medicines.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine. Dalacin vaginal cream can be used during pregnancy only under the supervision of a doctor.
Pregnancy
Dalacin vaginal cream may be used during the first trimester of pregnancy only if, in the doctor's opinion, it is absolutely necessary, as the data on the safety of use during the first three months of pregnancy are limited.
Breastfeeding
There are insufficient data on the penetration of clindamycin into breast milk.
In a breastfed infant, it is not possible to exclude the occurrence of severe side effects. Therefore, the doctor will decide whether the patient can take clindamycin and breastfeed at the same time.
Driving and using machines
No studies have been conducted on the effect of the medicine on the ability to drive and use machines.
Dalacin contains propylene glycol, cetyl stearyl alcohol, and benzyl alcohol
This medicine contains 250 mg of propylene glycol in each 5 g of cream (applicator content), which corresponds to 50 mg/g.
This medicine contains 160.5 mg of cetyl stearyl alcohol in each 5 g of cream (applicator content), which corresponds to 32.1 mg/g. Cetyl stearyl alcohol may cause local skin reactions (e.g., contact dermatitis).
This medicine contains 50 mg of benzyl alcohol in each 5 g of cream (applicator content), which corresponds to 10 mg/g. Benzyl alcohol may cause allergic reactions and mild local irritation.
3. How to use Dalacin
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
The recommended dose is 5 g of cream (100 mg of clindamycin), i.e., the contents of one applicator, administered vaginally, once a day, preferably before bedtime, for 7 consecutive days. Depending on the symptoms, the doctor may recommend a shorter, 3-day treatment period.
Method of administration
Before using the cream, the patient should always wash their hands.
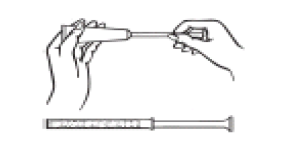
- Remove the cap from the tube.
- Screw one of the single-use applicators onto the tube in place of the removed cap.
- Holding the tube upside down, gently press the cream into the applicator.
- When the plunger stops moving, it means that the applicator has been filled.
- Unscrew the applicator from the tube and screw the cap back onto the tube.
- Lying on their back, the patient should firmly grasp the applicator and insert it into the vagina as far as possible without discomfort. Holding the applicator, they should slowly press the plunger until it stops, and the entire contents of the cream are administered.
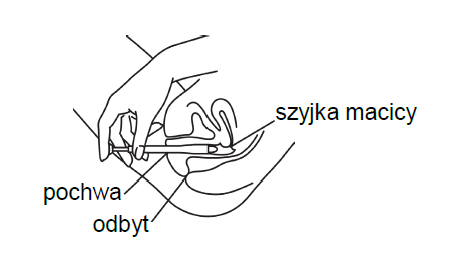
- Gently remove the applicator, wrap it in paper, and dispose of it in a waste container. The applicator should not be flushed down the toilet.
- After using the cream, the patient should wash their hands thoroughly.
Using more than the recommended dose of Dalacin
In case of overdose, the patient should immediately consult their doctor.
The patient should not use a higher dose without consulting their doctor.
Clindamycin phosphate used vaginally may be absorbed in amounts that cause systemic side effects (see sections 2 and 4). In case of overdose, the patient should consult their doctor, who will recommend appropriate treatment.
Missing a dose of Dalacin
The patient should not use a double dose to make up for a missed dose. If a dose is missed, the medicine should be used as soon as possible, unless it is almost time for the next dose.
Stopping the use of Dalacin
In case of doubts about the use of the medicine, the patient should consult their doctor or pharmacist.
The patient should not stop treatment without consulting their doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The safety of clindamycin in the form of vaginal cream has been evaluated in clinical trials conducted in non-pregnant women and pregnant women in the second and third trimesters of pregnancy. The following side effects have been observed in less than 10% of patients.
Very common(may affect more than 1 in 10 people):
- vaginal and vulvar candidiasis
Common(may affect up to 1 in 10 people):
- fungal infections, yeast infections
- headache, dizziness, taste disturbances
- upper respiratory tract infections
- abdominal pain, constipation, diarrhea, nausea, vomiting
- itching (in areas other than the application site), rash
- back pain
- urinary tract infections, glycosuria, proteinuria
- abnormal labor
- vaginitis, vulvitis, vaginal and vulvar disorders, menstrual disorders, vaginal and vulvar pain, metrorrhagia, discharge
Uncommon(may affect up to 1 in 100 people):
- bacterial infections
- allergic reactions
- vertigo of labyrinthine origin
- epistaxis
- local abdominal pain, bloating with gas, unpleasant breath odor
- urticaria, erythema
- painful or difficult urination
- trichomonal vaginitis, vaginitis, pelvic pain
- abnormal microbiological test results
Frequency not known(cannot be estimated from the available data):
- skin candidiasis
- hyperthyroidism
- pseudomembranous colitis, gastrointestinal disorders, dyspepsia
- maculopapular rash
- endometriosis
- inflammatory swelling, pain at the application site
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: + 48 22 49 21 301, fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Dalacin
The medicine should be stored out of sight and reach of children.
Do not store above 25°C. Do not freeze.
Do not use the medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Dalacin contains
- The active substance of the medicine is clindamycin. 5 g of cream (applicator content) contains 100 mg of clindamycin in the form of clindamycin phosphate.
- The other ingredients of the medicine are: sorbitan stearate, polysorbate 60, propylene glycol, stearic acid, cetyl stearyl alcohol, cetyl palmitate, liquid paraffin, benzyl alcohol, purified water.
What Dalacin looks like and what the pack contains
A laminated tube with a PE layer (LMDPE), sealed with a PP cap, with 7 single-use applicators made of PE, in a cardboard box.
Each applicator, once filled (one dose), contains approximately 5 g of cream (100 mg of clindamycin).
For more detailed information, the patient should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Hungary, the country of export:
Pfizer Kft.
1123 Budapest
Alkotás u. 53.
Hungary
Manufacturer:
Pfizer Service Company BVBA
Hoge Wei, 1930 Zaventem
Belgium
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Marketing authorization number in Hungary, the country of export: OGYI-T-958/10
Parallel import authorization number: 297/23
Date of leaflet approval: 22.12.2023
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Pfizer Kft.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DalacinDosage form: Suppositories, 100 mgActive substance: clindamycinPrescription requiredDosage form: Cream, 20 mg/g (2%)Active substance: clindamycinPrescription requiredDosage form: Cream, 20 mg/g (2%)Active substance: clindamycinPrescription required
Alternatives to Dalacin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Dalacin in Ukraine
Alternative to Dalacin in Spain
Online doctors for Dalacin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Dalacin – subject to medical assessment and local rules.







