
Clarelux

Ask a doctor about a prescription for Clarelux

How to use Clarelux
PATIENT INFORMATION LEAFLET: USER INFORMATION
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Clarelux, 500 micrograms/g, foam for the skin
Clobetasol propionate
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Clarelux and what is it used for
- 2. Important information before using Clarelux
- 3. How to use Clarelux
- 4. Possible side effects
- 5. How to store Clarelux
- 6. Contents of the pack and other information
1. What is Clarelux and what is it used for
Clarelux contains the active substance clobetasol propionate, which belongs to a group of medicines called topical corticosteroids. Clobetasol propionate is a corticosteroid with very strong effects.
Clarelux is available as a foam for application to the skin.
Clarelux is used for short-term treatment of steroid-responsive dermatoses of the scalp, such as scalp psoriasis, that do not respond satisfactorily to less potent corticosteroids.
2. Important information before using Clarelux
When not to use Clarelux:
- if the patient is allergic (hypersensitive) to clobetasol propionate, other corticosteroids, or any of the other ingredients of this medicine (listed in section 6);
- if the patient has skin infections: viral (e.g., herpes, shingles, chickenpox), bacterial (e.g., impetigo), fungal (caused by microscopic fungi), or parasitic;
- if the patient has burns, ulcers, or other skin diseases, such as rosacea, acne, perioral dermatitis, anal or genital itching;
- on any part of the body or face (including eyelids), except for the scalp;
- in children under 2 years of age.
Warnings and precautions
Before starting treatment with Clarelux, you should discuss it with your doctor or pharmacist.
You should stop using the medicine immediately and discuss it with your doctor if, during treatment, the disease worsens, as this may indicate the development of an allergic reaction (whose symptoms include skin rash, itching, or painless swelling), infection, or the need for other therapy.
If the disease recurs within a short period (within 2 weeks) after the end of treatment, you should not reuse Clarelux without first consulting your doctor, unless your doctor has previously recommended it. If the patient's disease has resolved, but after recurrence, the skin redness exceeds the previously treated area, and the patient feels burning of the skin, before resuming treatment, you should consult your doctor. It can be suspected that a rebound effect has occurred.
As with all topical corticosteroids, Clarelux may be absorbed through the skin and cause side effects, such as adrenal gland suppression - all possible side effects are listed in section 4. In connection with this:
- you should avoid long-term treatment with Clarelux;
- you should not apply Clarelux to a large area;
- treated areas should not be bandaged or covered, unless your doctor recommends it;
- you should not use Clarelux on wounds or ulcers;
- you should contact your doctor if you experience blurred vision or other vision disturbances.
You should inform your doctor if:
- you experience bone pain or worsening of existing bone symptoms, especially if Clarelux is used for a long time or repeatedly;
- you are taking other medicines containing corticosteroids or medicines intended to regulate the immune system (e.g., in autoimmune diseases or after organ transplantation). Concomitant use of Clarelux with these medicines may lead to severe infections.
- after 2 weeks of treatment, the patient's condition does not improve;
- an infection occurs, as it may be necessary to discontinue Clarelux;
- you start to experience vision disturbances, as this type of medicine may cause the development of cataracts and glaucoma.
You should wash your hands thoroughly after each use of the medicine.
In case of accidental contact of the medicine with the face or eyes, these areas should be rinsed with a large amount of water.
Children and adolescents
It is not recommended to use the medicine in children under 12 years of age.
Clarelux and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking, or have recently taken, and about medicines you plan to take.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Clarelux should not be used during pregnancy or breastfeeding, unless your doctor considers it absolutely necessary.
Driving and using machines
Clarelux should not affect your ability to drive or use machines.
Important information about some ingredients of Clarelux
Clarelux contains:
- 2145 mg of alcohol (ethanol) in each application, which may cause burning of damaged skin,
- 74 mg of propylene glycol in each application,
- cetyl alcohol and stearyl alcohol, which may cause local skin reactions (e.g., contact dermatitis).
3. How to use Clarelux
WARNINGS:
The container contains a flammable liquid under pressure.
Do not use or store near an open flame, ignition source, heat-generating materials, or electrical appliances in use.
Do not smoke while using or holding the container.
Clarelux should always be used as directed by your doctor. If you have any doubts, you should consult your doctor or pharmacist.
You should use Clarelux according to its intended use, i.e., only on the scalp. Do not swallow.
It is not recommended to squeeze the medicine directly onto your hand, as the foam will immediately start to melt when in contact with warm skin.
You should use Clarelux on the affected areas of the scalp, twice a day, once in the morning, once in the evening, according to the following instructions:
Note: To dose the foam correctly, you should hold the container upside down!
- 1. Shake the container vigorously.
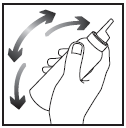
- 2. Invert the container upside downand press a small amount (the size of a walnut) directly onto the scalp or onto the cap of the container, onto the bottom or another cool surface, and then onto the scalp. You should apply a thin layer of Clarelux, as small as possible, to the affected skin. The exact amount needed depends on the size of the affected area. Do not apply to the eyelids or avoid the eye area, nose, and mouth. It is not recommended to squeeze Clarelux directly onto your hand, as the foam will immediately start to melt when in contact with warm skin.
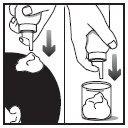
- 3. You should move the hair away from the foam and gently massage the foam into the scalp until it disappears and is absorbed. If necessary, repeat to apply the medicine to the entire affected area.
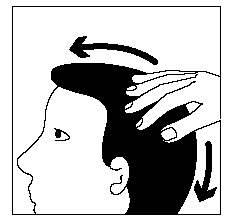
After each application of Clarelux, you should wash your hands thoroughly and remove any unused foam residue.
Do not apply Clarelux to the face. If the medicine accidentally gets into your eyes, nose, or mouth, you should rinse these areas with cold water immediately. The medicine may cause a stinging sensation. If the pain persists, you should consult your doctor.
You should not bandage or cover the treated areas, unless your doctor recommends it.
You should not wash or rinse the treated skin areas immediately after applying Clarelux.
You should not use more than 50 g of Clarelux per week.
Treatment should not last longer than 2 weeks. After this period, Clarelux may be used sporadically, as needed. Alternatively, your doctor may prescribe a weaker steroid to control your condition.
Using a higher dose of Clarelux than recommended
You should immediately inform your doctor if you have used Clarelux:
- in a dose higher than prescribed;
- for a longer period than recommended.
Missing a dose of Clarelux
You should apply the missed dose as soon as you remember, and then continue treatment as before. If you remember to apply the medicine only when it is time for the next dose, you should apply that dose as a single dose for that day, and then continue treatment as before (do not apply a double dose to make up for the missed dose). If you miss several doses, you should consult your doctor.
Stopping the use of Clarelux
You should not suddenly stop treatment, as it may be harmful. Your doctor may gradually stop treatment. Regular checks may be necessary.
If you have any further doubts about using this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Clarelux can cause side effects, although not everybody gets them.
You should stop using Clarelux and immediately consult your doctor if you experience hypersensitivity reactions, such as local irritation.
Side effects include:
Common side effects (may affect up to 1 in 10 people, but more than 1 in 100 people):
- Burning sensation
- Other skin reactions at the application site
Very rare side effects (may affect up to 1 in 10,000 people):
- Stinging or tingling sensation
- Eye irritation
- Vein swelling
- Skin irritation and tenderness
- Skin tension
- Itchy rash (contact dermatitis)
- Worsening of psoriasis
- Redness at the application site
- Itching and sometimes pain at the application site
- Possible presence of blood, protein, and nitrogen in the urine, which may be detected by your doctor
Additional side effects with unknown frequency (frequency cannot be estimated from the available data):
- Changes in hair growth (abnormal hair growth away from the application site and on unusual parts of the body)
- Changes in skin color
- Folliculitis (e.g., pain, sensation of heat, and redness)
- Rash around the mouth
- Redness and rash on the face
- Prolonged wound healing
- Effects on the eyes (cataracts, high eye pressure)
- Vision disturbances
Side effects due to long-term use with unknown frequency (frequency cannot be estimated from the available data):
- White spots on the skin (striae) and skin blood vessel dilation
- Similarly to other corticosteroids, Clarelux used in high doses and for a long period may cause Cushing's syndrome, which is characterized by a red, round face (so-called moon face), high blood pressure, weight gain, and changes in blood sugar and urine levels
- Long-term treatment with steroids may cause skin thinning
- Withdrawal reaction of topical steroid (rebound effect). If the medicine is used continuously for a long time, sudden discontinuation of treatment may cause a withdrawal reaction with some or all of the following symptoms: skin redness, which may include an area larger than the initially treated area, burning or stinging sensation, intense itching, skin peeling, and weeping open sores.
In rare cases, treatment of psoriasis with corticosteroids (or discontinuation of treatment) may cause worsening of lesions and development of a pustular form of the disease. After the end of corticosteroid treatment, scalp lesions may sometimes recur. Also, pre-existing infections may worsen if Clarelux is used not in accordance with the recommendations.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help gather more information on the safety of this medicine.
5. How to store Clarelux
- -The container contains a flammable liquid under pressure.
- -Do not store near an open flame, ignition source, heat-generating materials, or electrical appliances in use.
- -Do not expose to temperatures above 50°C or direct sunlight.
- -Do not pierce or burn the container, even if it is empty.
- -After the end of treatment, the container should be disposed of in a safe way.
Store in a place out of sight and reach of children.
Do not use Clarelux after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Do not store above 25°C. Do not store in the refrigerator. Store in an upright position.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Clarelux contains
The active substance of Clarelux is clobetasol propionate.
1 gram of foam contains 500 micrograms of clobetasol propionate.
The other ingredients are: anhydrous ethanol, purified water, propylene glycol, cetyl alcohol, stearyl alcohol, polysorbate 60, citric acid, potassium citrate, and a mixture of propane, n-butane, and isobutane gases.
What Clarelux looks like and contents of the pack
Clarelux is a white foam in a pressurized container.
The container contains 100 grams of foam.
For more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Greece, the country of export:
Pierre Fabre Farmaka A.E., A. Mesogeion 350, 153 41 Agia Paraskevi, Greece
Manufacturer:
Farmol Health Care S.r.L., Via del Maglio, 6, 23868 Valmadrera (LC), Italy
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Marketing authorization number in Greece, the country of export: 132338/20/04-08-2021
Parallel import authorization number: 88/24
This medicine is authorized in the Member States of the European Economic Area under the following names:
CLARELUX 500 micrograms/g cutaneous foam - Austria, Belgium, Czech Republic, France, Germany, Greece, Luxembourg, Netherlands, Poland, Portugal, Slovakia, United Kingdom, and Spain.
OLUX 500 micrograms/g cutaneous foam - Italy.
Date of approval of the leaflet: 06.03.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Pierre Fabre Farmaka A.E.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ClareluxDosage form: Foam, 500 mcg/gActive substance: clobetasolManufacturer: Farmol Health Care S.r.l.Prescription requiredDosage form: Foam, 500 micrograms/gActive substance: clobetasolPrescription requiredDosage form: Foam, 500 micrograms/gActive substance: clobetasolPrescription required
Alternatives to Clarelux in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Clarelux in Україна
Alternative to Clarelux in Іспанія
Online doctors for Clarelux
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Clarelux – subject to medical assessment and local rules.







