
Cisatracurium Kalceks
Ask a doctor about a prescription for Cisatracurium Kalceks

How to use Cisatracurium Kalceks
Package Leaflet: Information for the User
Cisatracurium Kalceks, 2 mg/ml, Solution for Injection/Infusion
Cisatracurium
Read the Package Leaflet Carefully Before Using the Medication, as it Contains
Important Information for the Patient.
Keep this Package Leaflet, so you can Read it Again if you Need to.
In Case of any Doubts, Consult your Doctor or Nurse.
If the Patient Experiences any Undesirable Effects, Including any Undesirable Effects not Listed in this Package Leaflet, Inform your Doctor or Nurse. See Section 4.
Table of Contents of the Package Leaflet:
- 1. What is Cisatracurium Kalceks and What is it Used for
- 2. Important Information Before Using Cisatracurium Kalceks
- 3. How to Use Cisatracurium Kalceks
- 4. Possible Undesirable Effects
- 5. How to Store Cisatracurium Kalceks
- 6. Contents of the Package and Other Information
1. What is Cisatracurium Kalceks and What is it Used for
Cisatracurium Kalceks Contains the Active Substance Cisatracurium. It Belongs to a Group of Medicines Called Muscle Relaxants.
Cisatracurium Kalceks is Used:
- to Relax Muscles During Surgical Procedures in Adults and Children Over 1 Month of Age, Including Cardiovascular Surgery;
- to Facilitate the Introduction of a Tube into the Trachea (Endotracheal Intubation), in Case the Patient Needs Respiratory Support;
- to Relax Muscles in Patients in Intensive Care Units.
To Obtain Additional Information About the Medication, Consult your Doctor.
2. Important Information Before Using Cisatracurium Kalceks
When Not to Use Cisatracurium Kalceks
Before Starting to Use Cisatracurium Kalceks, Discuss it with your Doctor or Nurse.
Warnings and Precautions
Before Starting to Take Cisatracurium Kalceks, Consult your Doctor or Nurse:
In Case of Doubts Whether any of the Above Situations Apply to the Patient, Consult your Doctor or Nurse Before Using Cisatracurium Kalceks.
Cisatracurium Kalceks and Other Medications
Tell your Doctor About all Medications the Patient is Currently Taking or has Recently Taken, as well as any Medications the Patient Plans to Take.
In Particular, Inform your Doctor if the Patient is Taking any of the Following Medications:
- Anesthetics (Used to Eliminate Sensation and Pain During Surgical Procedures);
- Other Medications Used to Relax Muscles;
- Antibiotics (Used to Treat Infections);
- Medications Used to Treat Heart Rhythm Disorders (Antiarrhythmic Medications);
- Medications Used to Treat High Blood Pressure;
- Diuretics, such as Furosemide;
- Medications Used to Treat Inflammatory Joint Conditions, such as Chloroquine or
- D-Penicillamine;
- Corticosteroids;
- Medications Used to Treat Seizures (Epilepsy), such as Phenytoin or Carbamazepine;
- Medications Used to Treat Mental Disorders, such as Lithium or Chlorpromazine (Also Used as an Antiemetic);
- Medications Containing Magnesium;
- Medications Used to Treat Alzheimer's Disease (Acetylcholinesterase Inhibitors, e.g., Donepezil).
Pregnancy, Breastfeeding, and Fertility
If the Patient is Pregnant or Breastfeeding, Thinks she may be Pregnant, or Plans to Have a Child, she Should Consult her Doctor Before Using this Medication.
It Cannot be Ruled out that Cisatracurium has a Negative Effect on the Breastfed Child, However, it Should not Have an Effect if Breastfeeding is Resumed After the Effect of the Substance has Passed. Cisatracurium is Quickly Eliminated from the Body. Women Should Refrain from Breastfeeding for 3 Hours After the End of Treatment.
Driving and Operating Machinery
If the Patient is in the Hospital for Only One Day, the Doctor will Determine the Time to Wait Before Leaving the Hospital or Driving a Vehicle. Driving too Soon After Surgery may be Dangerous.
3. How to Take Cisatracurium Kalceks
The Patient will Never Use this Medication Alone. The Medication will Always be Administered to the Patient by a Qualified Person.
Cisatracurium Kalceks can be Administered:
- as a Single Intravenous Injection (Rapid Intravenous Injection),
- as a Continuous Intravenous Infusion. The Medication is then Administered Slowly Over a Long Period.
The Doctor will Decide on the Method of Administration and the Dose of the Medication the Patient will Receive. This will Depend on:
- the Patient's Body Weight;
- the Required Duration and Degree of Muscle Relaxation;
- the Patient's Expected Response to the Medication.
The Medication must not be Used in Children Under 1 Month of Age.
Using More than the Recommended Dose of Cisatracurium Kalceks
The Medication will Always be Administered Under Medical Supervision. However, if the Patient Believes they have Received too Much Medication, they Should Immediately Inform their Doctor or Nurse.
4. Possible Undesirable Effects
Like all Medications, this Medication can Cause Undesirable Effects, although not Everybody gets them.
Allergic Reactions (May Occur in Less than 1 in 10,000 Patients)
In Case of an Allergic Reaction, Immediately Inform your Doctor or Nurse. Symptoms may Include:
- Sudden Wheezing, Chest Pain or Tightness;
- Swelling of the Eyelids, Face, Lips, Mouth, or Tongue;
- Hives or Itching on any Surface of the Body;
- Shock and Collapse.
In Case of the Following Symptoms, Inform your Doctor or Nurse:
Frequent (May Occur in Less than 1 in 10 Patients)
- Slow Heart Rate;
- Low Blood Pressure.
Uncommon (May Occur in Less than 1 in 100 Patients)
- Rash or Redness of the Skin;
- Wheezing or Coughing.
Very Rare (May Occur in Less than 1 in 10,000 Patients)
- Muscle Weakness or Pain.
Reporting Undesirable Effects
If any Undesirable Effects Occur, Including any Undesirable Effects not Listed in this Package Leaflet, Inform your Doctor or Nurse. Undesirable Effects can be Reported Directly to the Department of Monitoring of Undesirable Effects of Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Undesirable Effects can also be Reported to the Marketing Authorization Holder.
Reporting Undesirable Effects will Help to Gather more Information on the Safety of the Medication.
5. How to Store Cisatracurium Kalceks
Store the Medication in a Place that is Inaccessible to Children.
Store and Transport in a Cool Place (2°C – 8°C). Do not Freeze.
Store in the Original Package to Protect from Light.
Shelf Life After Dilution
Physical and Chemical Stability has been Demonstrated for at Least 24 Hours if Stored at 2-8°C and 25°C.
From a Microbiological Point of View, if the Method of Opening/Dilution does not Exclude the Risk of Microbiological Contamination, the Product should be Used Immediately. If not Used Immediately, the User is Responsible for the Storage Conditions.
Do not Use this Medication After the Expiration Date Stated on the Ampoule Label and on the Carton After the Word "EXP". The Expiration Date Refers to the Last Day of the Specified Month.
Medications should not be Disposed of via Wastewater or Household Waste. Ask your Pharmacist how to Dispose of Medications that are no Longer Needed. This will Help Protect the Environment.
6. Contents of the Package and Other Information
What Cisatracurium Kalceks Contains
- The Active Substance of the Medication is Cisatracurium (as Besylate). Each ml of Solution Contains 2 mg of Cisatracurium (as Cisatracurium Besylate). Each 2.5 ml Ampoule Contains 5 mg of Cisatracurium. Each 5 ml Ampoule Contains 10 mg of Cisatracurium. Each 10 ml Ampoule Contains 20 mg of Cisatracurium.
- Other Ingredients are: Benzenesulfonic Acid (to Adjust pH), Water for Injections.
What Cisatracurium Kalceks Looks Like and What the Package Contains
Clear, Colorless or Slightly Yellowish Solution, Free from Visible Particles.
2.5 ml, 5 ml, or 10 ml of Solution are Filled in Ampoules made of Colorless Glass Type I, with a Scored Point (Break Point).
The Ampoules are Marked with a Colored Ring, in a Different Color for Each Volume.
Five Ampoules are Placed in a PVC Overwrap, in a Carton.
Not all Pack Sizes may be Marketed.
Marketing Authorization Holder and Manufacturer
AS KALCEKS
Krustpils iela 71E
1057 Rīga
Latvia
This Medication is Authorized for Marketing in the Member States of the European Economic Area Under the Following Names:
Latvia
Cisatracurium Kalceks 2 mg/ml Šķīdums Injekcijām/Infūzijām
Austria
Cisatracurium Kalceks 2 mg/ml Injektions-/Infusionslösung
Belgium
Cisatracurium Kalceks 2 mg/ml, Solution Injectible/Pour Perfusion
Cisatracurium Kalceks 2 mg/ml, Oplossing Voor Injectie/Infusie
Cisatracurium Kalceks 2 mg/ml, Injektions-/Infusionslösung
Estonia
Cisatracurium Kalceks
France
CISATRACURIUM KALCEKS 2 mg/ml, Solution Injectabile/Pour Perfusion
Hungary
Cisatracurium Kalceks 2 mg/ml Oldatos Injekció/Infúzió
Ireland
Cisatracurium 2 mg/ml Solution for Injection/Infusion
Italy
Cisatracurio Kalceks
Lithuania
Cisatracurium Kalceks 2 mg/ml Injekcinis Ar Infuzinis Tirpalas
Poland
Cisatracurium Kalceks
Date of Last Revision of the Package Leaflet: 02/2022
------------------------------------------------------------------------------------------------------------------------
Information Intended for Healthcare Professionals Only:
Incompatibilities
Since Cisatracurium is Only Stable in Acidic Solutions, it must not be Mixed in the Same Syringe or Administered Through the Same Needle with Alkaline Solutions (e.g., Sodium Thiopental). Cisatracurium is not Compatible with Ketorolac Tromethamine or Propofol Emulsion for Injection.
Instructions for Use, Disposal, and Other Procedures
For Single Use Only.
The Medication should be Used Immediately After Opening the Ampoule.
Before Administration, Check the Appearance of the Solution. Do not Use the Medication if there are any Signs of Deterioration (e.g., Particles).
The Diluted Product Cisatracurium Kalceks is Physically and Chemically Stable for 24 Hours at 2-8°C and 25°C in a Concentration of 0.1 mg/ml in the Following Intravenous Solutions in Contact with Polypropylene or Polycarbonate Syringes, Polyethylene or PVC Tubing, and Polypropylene or PVC Infusion Bags:
- Sodium Chloride 9 mg/ml (0,9%) Solution for Injection;
- Glucose 50 mg/ml (5%) Solution for Injection;
- Sodium Chloride 1.8 mg/ml (0.18%) and Glucose 40 mg/ml (4%) Solution for Injection;
- Sodium Chloride 4.5 mg/ml (0.45%) and Glucose 25 mg/ml (2.5%) Solution for Injection.
Under Conditions Simulating Infusion Through a Y-Connector, Cisatracurium has been Shown to be Compatible with the Following Commonly Used Medications in the Perioperative Period: Alfentanil Hydrochloride, Droperidol, Fentanyl Citrate, Midazolam Hydrochloride, and Sufentanil Citrate.
If Other Medications are Administered Through the Same Indwelling Needle or Cannula as Cisatracurium Kalceks, it is Recommended to Flush the System with an Appropriate Volume of the Compatible Intravenous Solution, e.g., Sodium Chloride 9 mg/ml (0.9%) Solution for Injection.
Similarly, as with Other Intravenous Medications, if a Small Vein is Used for Injection, the Vein should be Flushed with an Appropriate Volume of Intravenous Solution, e.g., Sodium Chloride 9 mg/ml (0.9%) Solution for Injection, After Administration of Cisatracurium Kalceks.
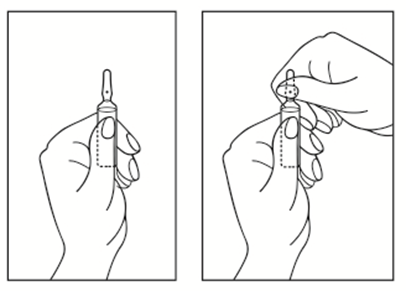
Instructions for Opening the Ampoule
- 1) Turn the Ampoule with the Colored Point Upwards. If there is any Solution in the Upper Part of the Ampoule, Gently Tap with your Finger to Get all the Solution to the Lower Part of the Ampoule.
- 2) Use Both Hands to Open; Holding the Lower Part of the Ampoule in one Hand, with the Other Hand, Break Off the Upper Part of the Ampoule in the Direction of the Colored Point (See the Diagram Below).
Any Unused Medicinal Product or Waste should be Disposed of in Accordance with Local Regulations.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAkciju sabiedriba "Kalceks"
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Cisatracurium KalceksDosage form: Solution, 5 mg/mlActive substance: cisatracuriumPrescription not requiredDosage form: Solution, 2 mg/mlActive substance: cisatracuriumPrescription not requiredDosage form: Solution, 2 mg/mlActive substance: cisatracuriumPrescription not required
Alternatives to Cisatracurium Kalceks in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cisatracurium Kalceks in Spain
Online doctors for Cisatracurium Kalceks
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cisatracurium Kalceks – subject to medical assessment and local rules.














