
Caspofungin Demo

Ask a doctor about a prescription for Caspofungin Demo

How to use Caspofungin Demo
PATIENT INFORMATION LEAFLET
Leaflet attached to the packaging: information for the user
Caspofungin DEMO 50 mg powder for concentrate for solution for infusion
Caspofungin
Read the contents of the leaflet carefully before using the medicine in a patient or their child,
as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, nurse or pharmacist.
- If the patient experiences any side effects, including any not listed in this leaflet, they should inform their doctor, nurse or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Caspofungin DEMO and what is it used for
- 2. Important information before using Caspofungin DEMO
- 3. How to use Caspofungin DEMO
- 4. Possible side effects
- 5. How to store Caspofungin DEMO
- 6. Contents of the pack and other information
1. What is Caspofungin DEMO and what is it used for
What is Caspofungin DEMO
Caspofungin DEMO contains a medicine called caspofungin, which belongs to a group of
antifungal medicines.
What is Caspofungin DEMO used for
Caspofungin DEMO is used to treat the following infections in children, adolescents and
adults:
- severe fungal infections of tissues and organs (called “invasive candidiasis”). This is an infection caused by fungal cells (yeast) called Candida. People who may develop this type of infection include patients who have recently had an operation or have a weakened immune system. The most common symptoms of this type of infection are fever and chills that do not go away with antibiotic treatment.
- fungal infections of the nose, sinuses or lungs (called “invasive aspergillosis”), if other antifungal medicines have not worked or have caused side effects. These infections are caused by a mold fungus called Aspergillus. People who may develop this type of infection include patients undergoing chemotherapy, after organ transplantation and with a weakened immune system.
- suspected fungal infection in people with fever and low white blood cell count, whose condition has not improved with antibiotic treatment. People who are at risk of developing a fungal infection include patients who have recently had an operation or have a weakened immune system.
How Caspofungin DEMO works
Caspofungin DEMO weakens the fungal cells and inhibits their normal growth. This stops the
infection from spreading and allows the body's natural defense mechanisms to completely eliminate it.
2. Important information before using Caspofungin DEMO
When not to use Caspofungin DEMO
- if the patient is allergic to caspofungin or any of the other ingredients of this medicine (listed in section 6).
In case of doubt before administration, consult a doctor, pharmacist or nurse.
Warnings and precautions
Before starting treatment with Caspofungin DEMO, discuss with your doctor, nurse or pharmacist:
- if the patient is allergic to any medicines;
- if the patient has ever had liver problems - a different dose of the medicine may be necessary;
- if the patient is taking cyclosporin (a medicine used to prevent transplant rejection or to suppress the immune system), as the doctor may order additional blood tests during treatment;
- if the patient has ever had any other health problems.
If any of the above statements apply to the patient (or are suspected), before using Caspofungin DEMO, consult a doctor, pharmacist or nurse.
Caspofungin DEMO may also cause severe skin reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).
Caspofungin DEMO and other medicines
Tell your doctor, nurse or pharmacist about all medicines the patient is taking, has recently taken or might take, including those obtained without a prescription, including herbal medicines, as Caspofungin DEMO
may affect the way other medicines work. Also, some other medicines may affect the way Caspofungin DEMO works.
If the patient is taking any of the following medicines, tell their doctor, nurse or pharmacist:
cytosporin or tacrolimus (medicines used to prevent transplant rejection or to suppress the immune system), as the doctor may order additional blood tests during treatment;
- certain medicines used to treat HIV infection, such as efavirenz or nevirapine;
- phenytoin or carbamazepine (used to treat seizures);
- dexamethasone (a steroid medicine);
- rifampicin (an antibiotic).
If any of the above statements apply to the patient (or are suspected), before using Caspofungin DEMO, tell their doctor, nurse or pharmacist.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant or plans to have a baby, they should consult their doctor or pharmacist before using this medicine.
- No studies have been conducted on the use of Caspofungin DEMO in pregnant women. During pregnancy, the medicine should only be used if the potential benefits of treatment outweigh the potential risk to the unborn baby.
- Women taking Caspofungin DEMO should not breastfeed.
Driving and using machines
There is no information to suggest that this medicine may affect the ability to drive or use machines.
3. How to use Caspofungin DEMO
Caspofungin DEMO will always be prepared and administered by medical personnel.
Caspofungin DEMO will be administered:
- once a day;
- by slow intravenous infusion (intravenous infusion);
- over a period of about 1 hour.
The duration of treatment and the daily dose of Caspofungin DEMO will be determined by the doctor. They will monitor the effectiveness of the medicine in the patient. In patients with a body weight over 80 kg, a different dose may be necessary.
Use in children and adolescents
The dose for children and adolescents may be different from the dose used in adults.
Using a higher dose of Caspofungin DEMO than recommended
The doctor will decide what daily dose of Caspofungin DEMO the patient needs and how long the treatment should last. If there is any doubt as to whether the patient has received too high a dose of Caspofungin DEMO, they should immediately consult their doctor or nurse.
In case of any further doubts about the use of this medicine, consult a doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following side effects are noticed, tell a doctor or nurse immediately, as medical attention may be necessary:
- rash, itching, feeling of warmth, swelling of the face, lips or throat or difficulty breathing - possible histamine reaction to the medicine;
- difficulty breathing with wheezing or worsening rash - possible allergic reaction to the medicine;
- cough, severe breathing difficulties - in adult patients with invasive aspergillosis, severe breathing difficulties may occur, which can lead to respiratory failure;
- rash, skin peeling, mucosal ulcers, hives, skin peeling over a large area.
As with all prescription medicines, some side effects can be serious. Consult a doctor for further information.
Other side effects that occur in adults include:
Common:may occur in up to 1 in 10 people:
- Decreased hemoglobin (decreased red blood cell count), decreased white blood cell count
- Decreased albumin (a type of protein) in the blood, decreased potassium or low potassium in the blood
- Headache
- Phlebitis
- Shortness of breath
- Diarrhea, nausea or vomiting
- Changes in the results of some blood laboratory tests (including increased liver function test results)
- Itching, rash, redness of the skin or excessive sweating
- Joint pain
- Chills, fever
- Itching at the injection site.
Uncommon:may occur in up to 1 in 100 people:
- Changes in the results of some blood laboratory tests (including blood clotting, platelet count, red and white blood cell count)
- Lack of appetite, increased fluid in the body, imbalance of salt levels in the body, high blood sugar, low calcium in the blood, high calcium in the blood, low magnesium in the blood, increased acidity of the blood
- Disorientation, feeling of agitation, insomnia
- Dizziness, weakness or numbness (especially of the skin), tremors, drowsiness, change in taste, feeling of numbness or tingling
- Blurred vision, increased tearing, swelling of the eyelids, yellowing of the whites of the eyes (sclera)
- Feeling of rapid or irregular heartbeat, rapid heartbeat, irregular heartbeat, abnormal heart rhythm, heart failure
- Flushing, hot flashes, high blood pressure, low blood pressure, redness of the skin along the vein, extremely sensitive to touch
- Constriction of the airways causing wheezing or cough, rapid breathing, shortness of breath that wakes you up from sleep, lack of oxygen in the blood, abnormal breathing sounds, crackling in the lungs, wheezing, stuffy nose, cough, sore throat
- Abdominal pain, pain in the upper abdomen, bloating, constipation, difficulty swallowing, dry mouth, indigestion, gas, stomach upset, swelling due to fluid accumulation in the abdominal cavity
- Decreased bile flow, liver enlargement, yellowing of the skin and/or whites of the eyes (jaundice), chemical or drug-induced liver damage, liver function disorders
- Abnormal skin changes, generalized itching, hives, rash, abnormal skin appearance, presence of red, often itchy patches on the hands and feet, and sometimes on the face and other parts of the body
- Back pain, pain in the arms or legs, bone pain, muscle pain, muscle weakness
- Worsening of kidney function, sudden worsening of kidney function
- Pain at the infusion site, changes at the infusion site (redness, hardening, pain, swelling, irritation, rash, hives, fluid leakage from the catheter into the tissues), phlebitis at the infusion site
- Increased blood pressure and changes in the results of some blood laboratory tests (including kidney function, electrolytes and blood clotting), increased levels of immunosuppressive drugs
- Feeling of discomfort in the chest, chest pain, feeling of body temperature change, general malaise, generalized pain, swelling of the face, swelling of the ankles, hands or feet, swelling, tenderness, feeling of fatigue.
Side effects in children and adolescents
Very common:may occur in more than 1 in 10 people:
- Fever.
Common:may occur in up to 1 in 10 people:
- Headache
- Rapid heartbeat
- Sudden flushing, low blood pressure
- Changes in the results of some blood laboratory tests (including increased liver function test results)
- Itching, rash
- Pain at the infusion site
- Chills
- Changes in the results of some blood laboratory tests.
Reporting side effects
If any side effects occur, including any not listed in this leaflet, tell a doctor, pharmacist or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices and Biocides
Al. Jerozolimskie 181C
PL-02 222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Caspofungin DEMO
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vial after (EXP). The expiry date refers to the last day of that month.
Store in a refrigerator (2°C to 8°C). It can also be stored at a temperature below 25°C for 1 month.
After preparation, Caspofungin DEMO should be used immediately, as it does not contain any preservatives. The medicine should only be prepared by trained medical personnel who have read all the instructions (see below “Instructions for reconstitution and dilution of Caspofungin DEMO”).
Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Caspofungin DEMO contains
- The active substance is caspofungin. Each vial contains 50 mg of caspofungin (as caspofungin acetate). The concentration after reconstitution will be 5.2 mg/mL.
- The other ingredients are: sucrose, mannitol, acetic acid and sodium hydroxide (see section 2 “Important information before using Caspofungin DEMO”).
What Caspofungin DEMO looks like and contents of the pack
This medicine is a sterile, white to off-white, lyophilized powder in a clear, colorless glass vial (type I) with a capacity of 10 mL, closed with a rubber stopper and sealed with an aluminum cap.
Each cardboard box contains one vial of powder.
Marketing authorization holder and manufacturer
Demo S.A. Pharmaceutical Industry
21st Km National Road Athens-Lamia
14568 Krioneri
Greece
T:+30 210 8161802, F:+30 210816158
This medicine is authorized in the Member States of the European Economic Area under the following names:
Cyprus: Caspofungin DEMO 50 mg powder for concentrate for solution for infusion
Germany: Caspofungin DEMO 50 mg powder for concentrate for solution for infusion
Greece: Caspofungin DEMO 50 mg powder for concentrate for solution for infusion
Ireland: Caspofungin 50 mg powder for concentrate for solution for infusion
United Kingdom: Caspofungin 50 mg powder for concentrate for solution for infusion
Netherlands: Caspofungin DEMO 50 mg powder for concentrate for solution for infusion
Spain: Caspofungina DEMO 50 mg powder for concentrate for solution for infusion EFG
Romania: Caspofungina DEMO 50 mg powder for concentrate for solution for infusion
Czech Republic: Caspofungin DEMO
Slovakia: Caspofungin DEMO 50 mg powder for concentrate for solution for infusion
Hungary: Caspofungin DEMO 50 mg powder for concentrate for solution for infusion
Finland: Caspofungin Demo S.A. 50 mg powder for concentrate for solution for infusion, solution
Sweden: Caspofungin Demo S.A.
Denmark: Caspofungin Demo S.A.
Norway: Caspofungin Demo S.A.
Poland: Caspofungin DEMO
Italy: Caspofungin DEMO
Date of last revision of the leaflet:
<------------------------------------------------------------------------------------------------------------------------>
Information intended for healthcare professionals only:
Instructions for reconstitution and dilution of Caspofungin DEMO:
Reconstitution of Caspofungin DEMO
DO NOT USE ANY SOLUTIONS CONTAINING GLUCOSE, as Caspofungin DEMO is not stable in glucose-containing solutions. DO NOT MIX OR ADMINISTER IN THE SAME INFUSION WITH OTHER MEDICINES, as there are no data on the co-administration of Caspofungin DEMO with other intravenous substances, excipients or other medicinal products.
The infusion solution should be inspected for particulate matter and discoloration.
Preparation of the diluted solution for infusion
- Sodium chloride 0.9%
- Sodium chloride 0.45%
- Sodium chloride 0.225%
- Ringer's solution with lactate
INSTRUCTIONS FOR ADMINISTERING THE MEDICINE TO ADULT PATIENTS
Step 1 Reconstitution of the vial contents
To reconstitute the powder, remove the vial from the refrigerator and allow it to reach room temperature, then aseptically add 10.5 mL of water for injection. The resulting concentration will be: 5.2 mg/mL.
The white to off-white, lyophilized powder should dissolve completely. Gently mix the contents until a clear solution is obtained. The reconstituted solution can be stored for up to 24 hours at a temperature not exceeding 25°C. The reconstituted solution should be inspected for particulate matter and discoloration before use. If the solution is cloudy or contains precipitate, do not use.
Step 2 Addition of the reconstituted Caspofungin DEMO to the infusion solution for the patient
To prepare the final infusion solution, the following solutions can be used: sodium chloride injection solution or Ringer's lactate injection solution. The infusion solution should be prepared by aseptically adding the appropriate volume of the reconstituted concentrate (as shown in the table below) to an infusion bag or bottle containing 250 mL of sodium chloride injection solution or Ringer's lactate injection solution. Infusions with a reduced volume of 100 mL can be used when medically necessary for daily doses of 50 mg or 35 mg. Do not use if the solution is cloudy or contains precipitate.
PREPARATION OF THE INFUSION SOLUTION FOR ADULTS
| DOSE* | Volume of reconstituted Caspofungin DEMO to be transferred to the infusion bag or bottle | Standard preparation (reconstituted Caspofungin DEMO added to 250 mL), final concentration | Reduced infusion volume (reconstituted Caspofungin DEMO added to 100 mL), final concentration |
| 50 mg | 10 mL | 0.20 mg/ mL | |
| 50 mg in reduced volume | 10 mL | 0.47 mg/ mL | |
| 35 mg in moderate liver impairment (from one 50 mg vial) | 7 mL | 0.14 mg/ mL | |
| 35 mg in moderate liver impairment (from one 50 mg vial) in reduced volume | 7 mL | 0.34 mg/ mL |
* To reconstitute the contents of each vial, 10.5 mL of water for injection should be used.
INSTRUCTIONS FOR ADMINISTERING THE MEDICINE TO CHILDREN AND ADOLESCENTS
Calculation of body surface area (BSA) to determine the dose in children and adolescents
Before preparing the infusion, calculate the patient's body surface area using the following formula: (Mosteller's formula)
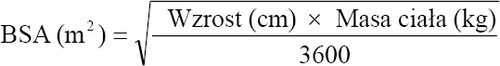
Mosteller RD: Simplified Calculation of Body Surface Area. N Engl J Med1987 Oct 22;317(17):
1098 (letter)
Preparation of the intravenous infusion containing a dose of 70 mg/m² for children and adolescents over 3 months of age (using a vial containing 50 mg of the product)
1. Determine the actual loading dose to be used in children and adolescents based on the body surface area (calculated as above) using the following equation: BSA (m²) x 70 mg/m² = Loading dose Maximum loading dose on Day 1 of therapy should not exceed 70 mg, regardless of the calculated dose for the individual patient.
- 1. Determine the actual loading dose to be used in children and adolescents based on the body surface area (calculated as above) using the following equation: BSA (m²) x 70 mg/m² = Loading dose Maximum loading dose on Day 1 of therapy should not exceed 70 mg, regardless of the calculated dose for the individual patient.
- 2. Remove the vial from the refrigerator and allow it to reach room temperature.
- 3. Aseptically add 10.5 mL of water for injection. The resulting solution can be stored for up to 24 hours at a temperature not exceeding 25°C. In this way, a concentration of 5.2 mg/mL is obtained in the vial.
- 4. From the vial, withdraw a volume of the solution equivalent to the calculated loading dose (step 1). Aseptically add this volume (mL) of the reconstituted Caspofungin DEMO to an infusion bag or bottle containing 250 mL of sodium chloride injection solution or Ringer's lactate injection solution. Alternatively, the specified volume (mL) of the reconstituted Caspofungin DEMO can be added to a smaller volume of sodium chloride injection solution or Ringer's lactate injection solution, without exceeding a final concentration of 0.5 mg/mL. The prepared infusion solution should be used within 24 hours if stored at a temperature not exceeding 25°C or within 48 hours if stored in a refrigerator at a temperature between 2°C and 8°C. Preparation of the intravenous infusion containing a dose of 50 mg/m² for children and adolescents over 3 months of age (using a vial containing 50 mg of the product)
- 1. Determine the actual daily maintenance dose to be used in children and adolescents based on the body surface area (calculated as above) using the following equation: BSA (m²) x 50 mg/m² = Daily maintenance dose Daily maintenance dose should not exceed 70 mg, regardless of the calculated dose for the individual patient.
- 2. Remove the vial from the refrigerator and allow it to reach room temperature.
- 3. Aseptically add 10.5 mL of water for injection. The resulting solution can be stored for up to 24 hours at a temperature not exceeding 25°C. In this way, a concentration of 5.2 mg/mL is obtained in the vial.
- 4. From the vial, withdraw a volume of the solution equivalent to the calculated daily maintenance dose (step 1). Aseptically add this volume (mL) of the reconstituted Caspofungin DEMO to an infusion bag or bottle containing 250 mL of sodium chloride injection solution or Ringer's lactate injection solution. Alternatively, the specified volume (mL) of the reconstituted Caspofungin DEMO can be added to a smaller volume of sodium chloride injection solution or Ringer's lactate injection solution, without exceeding a final concentration of 0.5 mg/mL. The prepared infusion solution should be used within 24 hours if stored at a temperature not exceeding 25°C or within 48 hours if stored in a refrigerator at a temperature between 2°C and 8°C.
Notes on preparation:
- a. The white to off-white powder should dissolve completely. Gently mix the contents until a clear solution is obtained.
- b. The reconstituted solution should be inspected for particulate matter and discoloration before use. If the solution is cloudy or contains precipitate, do not use.
- c. Caspofungin DEMO is prepared to allow for the administration of the full dose stated on the vial label (50 mg) after the removal of 10 mL from the vial.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterDEMO S.A. Pharmaceutical Industry
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Caspofungin DemoDosage form: Powder, 50 mgActive substance: caspofunginManufacturer: Adamed Pharma S.A. ELPEN Pharmaceutical Co.Inc. Pharmadox Healthcare, Ltd. Pharmathen S.A.Prescription requiredDosage form: Powder, 70 mgActive substance: caspofunginManufacturer: Adamed Pharma S.A. ELPEN Pharmaceutical Co.Inc. Pharmadox Healthcare, Ltd. Pharmathen S.A.Prescription requiredDosage form: Powder, 70 mgActive substance: caspofunginPrescription required
Alternatives to Caspofungin Demo in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Caspofungin Demo in Spain
Alternative to Caspofungin Demo in Ukraine
Online doctors for Caspofungin Demo
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Caspofungin Demo – subject to medical assessment and local rules.














