

Canespor Onihoset

Ask a doctor about a prescription for Canespor Onihoset

How to use Canespor Onihoset
Package Leaflet: Information for the Patient
CANESPOR ONYCHOSET, 10 mg + 400 mg/g, ointment
(Bifonazole + Urea)
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet
- 1. What is Canespor Onychoset and what is it used for
- 2. Important information before using Canespor Onychoset
- 3. How to use Canespor Onychoset
- 4. Possible side effects
- 5. How to store Canespor Onychoset
- 6. Contents of the pack and other information
1. What is Canespor Onychoset and what is it used for
Canespor Onychoset is an antifungal medicine, in the form of an ointment. The medicine contains the active substance bifonazole with a broad spectrum of antifungal activity and urea, which facilitates the penetration of bifonazole into the nail and enhances its effect.
Canespor Onychoset is indicated for the local treatment of fungal nail infections of the hands and feet, including painless removal of the nail and simultaneous antifungal treatment.
2. Important information before using Canespor Onychoset
When not to use Canespor Onychoset
- if you are allergic to the active substances - bifonazole and urea - or to any of the other ingredients of this medicine listed in section 6.
Warnings and precautions
Before starting to use Canespor Onychoset, discuss it with your doctor or pharmacist.
Patients who have previously experienced hypersensitivity reactions to other antifungal imidazole derivatives, e.g. econazole, clotrimazole, miconazole, should use bifonazole-containing medicines with caution.
If the symptoms of the disease persist or do not improve after stopping treatment, you should contact your doctor.
Avoid contact of the medicine with the eyes. Do not swallow.
Children and adolescents
In infants and small children, the medicine can only be used on the advice and under the supervision of a doctor.
Canespor Onychoset and other medicines
Using Canespor Onychoset with warfarin increases the risk of bleeding. If Canespor Onychoset and warfarin are used together, the patient should be under close medical supervision.
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and about any medicines you plan to use.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not use Canespor Onychoset during the first trimester of pregnancy.
If your doctor decides that you need to use Canespor Onychoset during breastfeeding, you should stop breastfeeding.
Driving and using machines
Canespor Onychoset has no influence or negligible influence on the ability to drive and use machines.
Canespor Onychoset contains lanolinand may cause local skin reactions, e.g. contact dermatitis.
3. How to use Canespor Onychoset
This medicine should always be used as directed by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
Recommended dose
Canespor Onychoset is applied once a day, applying a thin layer of ointment to the entire surface of the diseased nail.
Method of application
- 1. Wash your foot or hand in warm water, dry thoroughly.
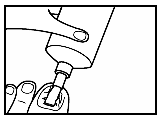
- 2. Squeeze out a strip of ointment from the tube, the length of which is sufficient to cover the infected nail. Apply the ointment to the diseased nail. Do not rub in.
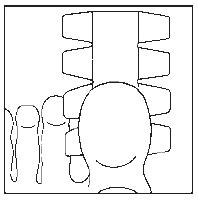
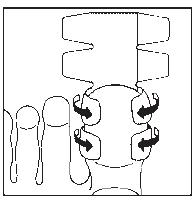
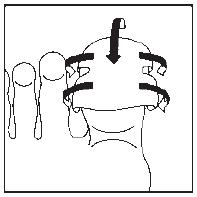
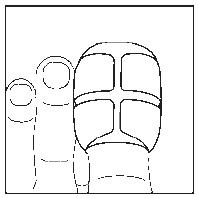
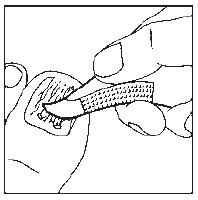
- 3. Cover the nail with half of the plaster. (The drawings show the bottom of the foot.) Note! For small nails, the plaster sheet can be divided into smaller parts.
- 4. Stick the sides of the plaster coated with adhesive to the underside of the finger.
- 5. Cover the underside of the finger with the second half of the plaster.
- 6. In this way, the nail will be completely covered. Leave the dressing on for 24 hours. (The drawing shows the top of the foot.)
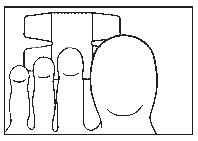
- 7. After 24 hours, remove the plaster, then soak your hand or foot in warm water for 10 minutes and dry thoroughly.
- 8. Using a nail file, carefully remove the softened layer of the infected nail.
- 9. Then dry the nails thoroughly and reapply the medicine and dressing. The medicine only works on infected nail surfaces, leaving healthy parts unchanged. Usually, it is not necessary to protect the surrounding healthy skin. However, if irritation occurs, protect the surrounding skin, e.g. by applying zinc paste. The size of the plaster in the kit corresponds to the size of the nails. Duration of treatment Treatment with Canespor Onychoset should last until all infected layers of the nail are removed. This usually takes from 7 to 14 days. After removing the infected layers of the nail, continue treatment with Canespor cream, applying it once a day for the next 4 weeks. The amount of ointment in the package is sufficient for 30 applications, i.e. you can treat 3 infected nails for 10 days. If the plasters are used up before the ointment, you can purchase another waterproof plaster and adjust its size to the size of the nails.
Use in children and adolescents
In infants and small children, the medicine can only be used on the advice and under the supervision of a doctor.
There are no data on the use in newborns.
Using more than the recommended dose of Canespor Onychoset
There is no risk of acute overdose.
Missing a dose of Canespor Onychoset
Do not use a double dose to make up for a missed dose.
Use the next dose as soon as possible.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Canespor Onychoset can cause side effects, although not everybody gets them.
Frequency not known (cannot be estimated from the available data):
contact dermatitis, skin maceration, skin peeling, nail disorders, nail discoloration, redness, irritation, pain at the application site, limb pain, itching, rash.
Side effects usually disappear after treatment is stopped.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Canespor Onychoset
Keep this medicine out of the sight and reach of children.
There are no special precautions for storage.
Do not use this medicine after the expiry date stated on the carton. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Canespor Onychoset contains
- The active substance of the medicine is bifonazole and urea.
- The other ingredients are: white wax, white petrolatum, lanolin.
What Canespor Onychoset looks like and contents of the pack
Canespor Onychoset is an ointment.
The medicine is available in a kit containing:
- a tube with 10 g of ointment,
- 15 plasters,
- a nail file. The whole is placed in a cardboard box.
Marketing Authorisation Holder
Bayer Sp. z o.o.
Al. Jerozolimskie 158
02-326 Warsaw
Manufacturer
GP Grenzach Produktions GmbH
Emil-Barell-Str. 7
79639 Grenzach-Wyhlen
Germany
To obtain more detailed information, please contact the local representative of the Marketing Authorisation Holder:
Poland
Bayer Sp. z o.o.
Aleje Jerozolimskie 158
02-326 Warsaw
Phone: +48 22 572 3500
Fax: +48 22 572 35 00
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGP Grenzach Produktions GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Canespor OnihosetDosage form: Cream, 10 mg/gActive substance: bifonazoleManufacturer: GP Grenzach Produktions GmbHPrescription not requiredDosage form: Cream, 10 mg/gActive substance: bifonazolePrescription not requiredDosage form: Cream, 10 mg/gActive substance: bifonazolePrescription not required
Alternatives to Canespor Onihoset in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Canespor Onihoset in Ukraine
Alternative to Canespor Onihoset in Spain
Online doctors for Canespor Onihoset
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Canespor Onihoset – subject to medical assessment and local rules.







