

Candepres

Ask a doctor about a prescription for Candepres

How to use Candepres
Leaflet accompanying the packaging: patient information
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Candepres (Candesartan 1A Pharma)
32 mg, tablets
Candesartan cilexetil
Candepres and Candesartan 1A Pharma are different trade names for the same medicine.
It is essential to carefully read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
- The leaflet should be kept so that it can be read again if necessary.
- In case of any doubts, the patient should consult a doctor or pharmacist.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Candepres and what is it used for
- 2. Important information before taking Candepres
- 3. How to take Candepres
- 4. Possible side effects
- 5. How to store Candepres
- 6. Contents of the packaging and other information
1. What is Candepres and what is it used for
The medicine is called Candepres. It contains the active substance - candesartan cilexetil, which belongs to a group of so-called angiotensin II receptor antagonists. Candepres causes relaxation and dilation of blood vessels, which helps to lower blood pressure. It also makes it easier for the heart to pump blood to all parts of the body.
This medicine is used for:
- treatment of high blood pressure (hypertension) in adults and children and adolescents from 6 to 18 years old;
- treatment of adult patients with heart failure, with reduced heart muscle function, when ACE inhibitors cannot be used or as adjunctive therapy during treatment with ACE inhibitors, when symptoms of the disease persist despite treatment, and mineralocorticoid receptor antagonists (MRA) cannot be used. ACE inhibitors and MRA are medicines used to treat heart failure.
2. Important information before taking Candepres
When not to take Candepres
- if the patient is allergic to candesartan cilexetil or any of the other ingredients of this medicine (listed in section 6);
- if the patient is pregnant for more than 3 months (it is also better not to take Candepres in early pregnancy - see the section on "Pregnancy and breastfeeding");
- if the patient has severe liver disease or bile duct obstruction (a disorder of bile drainage from the gallbladder);
- if the patient has diabetes or kidney problems and is taking a blood pressure-lowering medicine containing aliskiren;
- in children under 1 year of age.
In case of doubt as to whether any of the above situations apply to the patient, they should consult a doctor or pharmacist before taking Candepres.
Warnings and precautions
Before starting treatment with Candepres, the patient should discuss it with their doctor or pharmacist,
if the patient:
- has heart, liver, or kidney problems, or is undergoing dialysis;
- has recently undergone a kidney transplant;
- has vomiting or has recently had severe vomiting or has diarrhea;
- has adrenal gland disease (Conn's syndrome, also known as primary hyperaldosteronism);
- has low blood pressure;
- has had a stroke in the past;
- is taking any of the following medicines for high blood pressure:
- an ACE inhibitor (e.g., enalapril, lisinopril, ramipril), especially if the patient has kidney disease caused by diabetes
- aliskiren
- is taking an ACE inhibitor along with a mineralocorticoid receptor antagonist (MRA) for heart failure treatment (see "Candepres and other medicines").
If the patient is pregnant (or may become pregnant), they must inform their doctor.
Candepres is not recommended in early pregnancy and is contraindicated after the 3rd month of pregnancy, as its use at this time may be very harmful to the fetus (see the section on pregnancy).
The doctor may recommend regular checks of kidney function, blood pressure, and electrolyte levels (e.g., potassium). See also the information in the section "When not to take Candepres".
If any of the above points apply to the patient, the doctor may recommend more frequent check-ups and performance of certain tests.
If the patient is to undergo surgery, they should inform their doctor or dentist about taking Candepres, as the medicine used in combination with certain anesthetics may cause excessive lowering of blood pressure.
Children and adolescents
The effect of candesartan cilexetil in children has been studied. The doctor will provide further information.
Candepres should not be used in children under 1 year of age due to the risk of harmful effects on developing kidneys.
Candepres and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Candepres may affect the action of some other medicines, and some medicines may affect the action of Candepres. If the patient is taking certain medicines, the doctor may consider it necessary to perform periodic blood tests.
Particular attention should be paid to informing the doctor about taking any of the following medicines, as the doctor may need to change the dose of the medicine and/or recommend other precautions:
- other blood pressure-lowering medicines, including beta-blockers, diazoxide, and ACE inhibitors, such as enalapril, captopril, lisinopril, or ramipril;
- non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, naproxen, diclofenac, celecoxib, or etoricoxib (pain-relieving and anti-inflammatory medicines);
- acetylsalicylic acid, if taken in a dose greater than 3 g per day (a pain-relieving and anti-inflammatory medicine);
- potassium supplements or potassium-containing salt substitutes (medicines that increase the amount of potassium in the blood);
- heparin (a medicine that prevents blood clotting);
- co-trimoxazole (an antibiotic), also known as trimethoprim with sulfamethoxazole;
- diuretics;
- lithium (a medicine used to treat mental disorders);
- an ACE inhibitor or aliskiren (see also the information in the sections "When not to take Candepres" and "Warnings and precautions");
- an ACE inhibitor along with certain other medicines used to treat heart failure, such as mineralocorticoid receptor antagonists (e.g., spironolactone, eplerenone).
Candepres with food, drink, and alcohol
- Candepres can be taken with or without food.
- If the patient is taking Candepres, they should consult their doctor before consuming alcohol. Alcohol may cause dizziness or fainting.
Pregnancy and breastfeeding
Pregnancy
If the patient suspects that they are (or may be) pregnant, they must inform their doctor. The doctor will usually advise stopping Candepres before becoming pregnant or as soon as possible after pregnancy is confirmed and recommend taking another medicine instead. Using Candepres in early pregnancy is not recommended, and it should not be used after the 3rd month of pregnancy, as it may be very harmful to the fetus.
Breastfeeding
The patient should inform their doctor about breastfeeding or intending to breastfeed. Taking Candepres is not recommended during breastfeeding. For patients planning to breastfeed, especially newborns or premature babies, the doctor may choose another suitable medicine.
Driving and using machines
Some people taking Candepres may feel tired or dizzy. In such cases, they should not drive vehicles, use tools, or operate machines.
Candepres contains lactose
If the patient has previously been diagnosed with intolerance to some sugars, they should contact their doctor before taking the medicine.
Candepres contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means it is considered "sodium-free".
3. How to take Candepres
This medicine should always be taken as directed by the doctor. In case of doubt, the patient should consult their doctor or pharmacist. It is essential to take the medicine every day.
Candepres can be taken with or without food.
The tablets should be swallowed with water.
The tablets should be taken daily at approximately the same time. This will help the patient remember to take the medicine.
Dividing the tablet
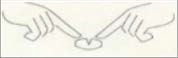
If necessary, the tablet can be divided into equal doses. To do this, the tablet should be placed on a hard, smooth surface (e.g., a table or plate) with the dividing line facing up, then briefly and firmly pressed with two index fingers (or thumbs) on both sides of the dividing line.
High blood pressure
- The recommended dose of Candepres is 8 mg once daily. The doctor may increase the dose to 16 mg once daily, and then to 32 mg once daily, depending on the response to treatment.
- Some patients, e.g., those with liver or kidney problems, or those who have recently lost a large amount of fluid (due to vomiting or diarrhea, or taking diuretics), may be prescribed a lower initial dose by their doctor.
- In black patients, the response to this type of medicine may be weaker if it is used alone (in so-called monotherapy). A higher dose of the medicine may be necessary.
Use in children and adolescents with high blood pressure
Children from 6 to 18 years old:
The recommended initial dose is 4 mg once daily.
- Patients with a body weight of <50 kg: in patients who do not achieve adequate blood pressure control, the doctor may increase dose to a maximum of 8 mg once daily.< li>
- Patients with a body weight of ≥50 kg: in patients who do not achieve adequate blood pressure control, the doctor may increase the dose to 8 mg once daily, and then to 16 mg once daily if necessary.
Heart failure
- The recommended initial dose of Candepres is 4 mg once daily. The doctor may double the dose of the medicine at intervals of at least 2 weeks up to a dose of 32 mg once daily. Candepres can be taken with other medicines used to treat heart failure, and the doctor will decide which treatment is most suitable for the patient.
Taking a higher dose of Candepres than recommended
In case of taking a higher dose of Candepres than recommended, the patient should immediately consult their doctor or pharmacist.
Missing a dose of Candepres
The patient should not take a double dose to make up for a missed dose. They should take the next dose at the usual time.
Stopping treatment with Candepres
If the patient stops taking Candepres, their blood pressure may rise again.
Therefore, the patient should not stop taking the medicine without consulting their doctor.
In case of any further doubts about taking this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Candepres can cause side effects, although not everybody gets them.
It is essential for the patient to know which side effects may occur.
In case of any of the following allergic reactions, the patient should stop taking Candepres and seek medical help immediately:
- difficulty breathing with swelling of the face, lips, tongue, and/or throat, with or without swelling;
- swelling of the face, lips, tongue, and/or throat, which may cause difficulty swallowing;
- severe itching of the skin (with a rash).
Candepres may cause a decrease in the number of white blood cells, which can weaken the body's resistance to infections. The patient may feel tired, and an infection or fever may occur. In such cases, the patient should contact their doctor, who will order blood tests to check if Candepres is affecting their blood (agranulocytosis).
Other possible side effects
Common(may occur in less than 1 in 10 people taking the medicine):
- dizziness, feeling of spinning
- headache
- respiratory tract infections
- low blood pressure (which may cause fainting or dizziness)
- changes in blood test results:
- increased potassium levels in the blood, especially in patients with kidney problems or heart failure. If the potassium level is very high, the patient may feel tired, weak, have irregular heartbeat, or tingling.
- effects on kidney function, especially in patients with kidney problems or heart failure. Very rarely, kidney failure may occur.
Very rare(may occur in less than 1 in 10,000 people taking the medicine):
- swelling of the face, lips, tongue, and/or throat
- decrease in the number of red or white blood cells. The patient may feel tired, and an infection or fever may occur.
- skin rash, hives
- itching
- back pain, joint and muscle pain
- changes in liver function, including hepatitis. The patient may feel tired, and jaundice or flu-like symptoms may occur.
- nausea
- changes in blood test results:
- decreased sodium levels in the blood. If the sodium level is very low, the patient may feel weak, lack energy, or have muscle cramps.
- cough.
Frequency not known(frequency cannot be estimated from the available data):
- diarrhea.
Additional side effects in children and adolescents
It appears that the side effects in children treated for high blood pressure are similar to those observed in adults but occur more frequently. Sore throat, as a side effect, occurs very frequently in children, and runny nose, fever, and rapid heartbeat occur frequently. These side effects were not reported in adults.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Candepres
The medicine should be stored out of sight and reach of children.
It should be stored in its original packaging to protect it from moisture.
The medicine should not be used after the expiry date stated on the packaging.
The expiry date stated on the packaging is the last day of the specified month.
Translation of some information on the immediate packaging:
Ch.-B./Verwendbar bis: siehe Prägung– batch number /expiry date – see embossing.
Medicines should not be disposed of via wastewater or household waste containers. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Candepres contains
- The active substance is candesartan cilexetil. Each tablet contains 32 mg of candesartan cilexetil.
- The other ingredients are: lactose monohydrate, cornstarch, povidone K 30, carrageenan, sodium croscarmellose, magnesium stearate, red iron oxide (E 172), titanium dioxide (E 171).
What Candepres looks like and what the packaging contains
Candepres is a pink, speckled, round, biconvex tablet with the symbol "32" embossed on one side and a dividing line on the other side.
Blister packs in a cardboard box: 30, 60 tablets.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Austria, the country of export:
1A Pharma GmbH
1020 Vienna, Austria
Manufacturer:
Lek Pharmaceuticals d.d., Verovśkova 57, 1526 Ljubljana, Slovenia
Lek Pharmaceuticals d.d., Trimlini 2D, 9220 Lendava, Slovenia
Lek S.A., ul. Domaniewska 50 C, 02-672 Warsaw, Poland
Salutas Pharma GmbH, Otto-von-Guericke-Allee 1, 39179 Barleben, Germany
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Austrian marketing authorization number:1-28445
Parallel import authorization number: 55/22 Date of leaflet approval: 19.01.2022
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)1A Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CandepresDosage form: Tablets, 8 mgActive substance: candesartanPrescription requiredDosage form: Tablets, 16 mgActive substance: candesartanPrescription requiredDosage form: Tablets, 8 mgActive substance: candesartanPrescription required
Alternatives to Candepres in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Candepres in Spain
Alternative to Candepres in Ukraine
Online doctors for Candepres
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Candepres – subject to medical assessment and local rules.










