

Bupivacainum hidrohloricum Vzf 0,5%

Ask a doctor about a prescription for Bupivacainum hidrohloricum Vzf 0,5%

How to use Bupivacainum hidrohloricum Vzf 0,5%
Leaflet attached to the packaging: patient information
BUPIVACAINUM HYDROCHLORICUM WZF 0.5%, 5 mg/ml, solution for injection
Bupivacaine hydrochloride
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- You should consult a doctor, pharmacist, or nurse if you have any doubts.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If you experience any side effects, including any possible side effects not listed in the leaflet, you should tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Bupivacainum hydrochloricum WZF 0.5% and what is it used for
- 2. Important information before using Bupivacainum hydrochloricum WZF 0.5%
- 3. How to use Bupivacainum hydrochloricum WZF 0.5%
- 4. Possible side effects
- 5. How to store Bupivacainum hydrochloricum WZF 0.5%
- 6. Contents of the packaging and other information
1. What is Bupivacainum hydrochloricum WZF 0.5% and what is it used for
The medicine contains bupivacaine hydrochloride, which belongs to a group of local anesthetics with an amide structure. It has a long-lasting anesthetic and analgesic effect. After administration, the medicine numbs specific parts of the body, so the patient does not feel pain.
The medicine is used:
- during operations and procedures under anesthesia (called infiltration, peripheral nerve, and epidural) - in adults and children over 12 years old;
- for the treatment of acute pain in adults, infants, and children from 1 year of age.
2. Important information before using Bupivacainum hydrochloricum WZF 0.5%
When not to use Bupivacainum hydrochloricum WZF 0.5%:
- in the method of anesthesia called Bier's block (administration of bupivacaine into a vein in a limb to induce anesthesia).
Warnings and precautions
Before starting treatment with Bupivacainum hydrochloricum WZF 0.5%, you should consult a doctor or nurse.
It is very important to tell your doctor about all health problems, especially:
Administration of the medicine in the eye area may cause permanent muscle function disorders.
Bupivacainum hydrochloricum WZF 0.5% is administered by anesthesiologists. During the administration of the medicine, they will provide the patient with proper care, and in case of problems, they will administer oxygen therapy (oxygen administration) and take other appropriate actions to maintain vital functions.
The safety and efficacy of Bupivacainum hydrochloricum WZF 0.5% have not been established in children under 12 years of age for the purpose of numbing specific parts of the body during surgical procedures.
The safety and efficacy of the medicine have not been established in children under 1 year of age.
Other medicines and Bupivacainum hydrochloricum WZF 0.5%
You should tell your doctor about all medicines you are currently taking or have recently taken, as well as medicines you plan to take.
In particular, you should tell your doctor if you are taking medicines for heart rhythm disorders, such as amiodarone. Other anesthetics (e.g., lidocaine) may enhance the effect of bupivacaine.
Pregnancy and breastfeeding
In pregnancy, during breastfeeding, or if you suspect you are pregnant, or if you plan to become pregnant, you should consult a doctor before using this medicine.
The use of the medicine during pregnancy and breastfeeding will be decided by a doctor.
Driving and using machines
Depending on the dose administered, local anesthetics may have a slight effect on the ability to drive vehicles and operate machinery. Therefore, it is not recommended to perform these activities on the day of anesthesia.
Bupivacainum hydrochloricum WZF 0.5% contains sodium
The medicine contains 3.15 mg of sodium in each ml of solution.
Ampoules of 10 ml: the medicine contains 31.5 mg of sodium (the main component of table salt) in 10 ml/ampoule.
This corresponds to 1.58% of the maximum recommended daily dose of sodium in the diet for adults.
Vials of 20 ml: the medicine contains 63 mg of sodium (the main component of table salt) in 20 ml/vial. This corresponds to 3.15% of the maximum recommended daily dose of sodium in the diet for adults.
The medicine may be diluted - see below "Preparation of Bupivacainum hydrochloricum WZF 0.5% for administration and method of administration". The sodium content from the diluent should be taken into account when calculating the total sodium content in the prepared dilution of the medicine. To obtain accurate information about the sodium content in the solution used for dilution, you should consult the leaflet of the diluent used.
Patients with reduced kidney function and those controlling their sodium intake should inform their doctor about this.
3. How to use Bupivacainum hydrochloricum WZF 0.5%
- The medicine is administered by a doctor, usually an anesthesiologist.
- The dose of the medicine may vary for individual patients. The dose will be determined by a doctor based on the patient's age, weight, health status, and the duration of the operation. A doctor will provide detailed information about the dosing of the medicine.
- Bupivacainum hydrochloricum WZF 0.5% is intended for adults and children over 12 years old for:
- infiltration anesthesia (injection of the medicine into the selected area where the surgical procedure will be performed);
- peripheral nerve anesthesia (administration of the medicine into the area of the nerve responsible for sensation in the area where the surgical procedure will be performed);
- epidural anesthesia (administration of the medicine into the so-called epidural space in the appropriate segment of the spine, if anesthesia of the lower part of the body is required).
- Bupivacainum hydrochloricum WZF 0.5% is intended for adults, infants, and children from 1 year of age for the treatment of pain.
Use of a higher dose of Bupivacainum hydrochloricum WZF 0.5% than recommended
The medicine is administered by a doctor, so it is unlikely that the patient will receive more medicine than they should.
If symptoms of overdose occur, the doctor will administer appropriate treatment.
Initially, the following symptoms of overdose are observed:
- numbness of the mouth and around the mouth;
- numbness of the tongue;
- dizziness;
- ringing in the ears, excessive sharpness of hearing (hyperacusis);
- vision disorders;
- speech disorders;
- muscle spasms. Severe symptoms of overdose (which occur very rarely) include: seizures, loss of consciousness, significant decrease in blood pressure, slowing of heart rate, arrhythmia, cardiac arrest, respiratory arrest. Due to increased muscle activity and respiratory disorders during seizures, hypoxia (decreased tissue oxygenation) and hypercapnia (increased carbon dioxide levels in the blood) occur quickly. Acid-base balance disorders, increased potassium levels in the blood, and decreased tissue oxygenation enhance the toxic effects of local anesthetics. The medical staff will take appropriate action.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effects of bupivacaine are due to overdose or unintentional intravascular administration of the medicine.
If the patient experiences the first symptoms of hypersensitivity (e.g., swelling of the face, lips, tongue, throat, causing difficulty breathing or swallowing), they should immediately tell their doctor. Such symptoms are rare. The doctor will then assess the severity of the symptoms and decide on further action.
Very common (more than 1 in 10 people) side effects include:
- hypotension (significant decrease in blood pressure);
- nausea.
Common (less than 1 in 10 people) side effects include:
- paresthesia (tingling, pricking, burning);
- dizziness;
- bradycardia (slow heart rate);
- hypertension (high blood pressure);
- vomiting;
- urinary retention.
Uncommon (less than 1 in 100 people) side effects include:
- symptoms of central nervous system toxicity (seizures, feeling of numbness around the mouth, numbness of the tongue, excessive sharpness of hearing, vision disorders, loss of consciousness, muscle tremors, dizziness, ringing in the ears, speech disorders).
Rare (less than 1 in 1,000 people) side effects include:
- neuropathy (symptoms: tingling, numbness, weakness of the innervated muscle);
- peripheral nerve damage (sensation disorders);
- arachnoiditis (one of the meninges);
- double vision;
- cardiac arrest, arrhythmia (irregular heartbeat);
- respiratory depression (severe breathing difficulties).
Reporting side effects
If you experience any side effects, including any possible side effects not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of the medicine.
5. How to store Bupivacainum hydrochloricum WZF 0.5%
Store ampoules and vials in the outer packaging to protect them from light, at a temperature below 25°C. Do not freeze.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the box, ampoule, or vial. The expiry date refers to the last day of the specified month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
The medicine does not contain preservatives. After the first dose is taken, the unused contents of the vial should be destroyed within 24 hours.
After taking a dose of the medicine from the ampoule, the remaining contents of the ampoule should be destroyed immediately.
Medicines should not be thrown into the sewage system or household waste containers. You should ask your pharmacist how to dispose of medicines that are no longer used. This will help protect the environment.
6. Contents of the packaging and other information
What does Bupivacainum hydrochloricum WZF 0.5% contain?
- The active substance of the medicine is bupivacaine hydrochloride. Each ml of the solution for injection contains 5 mg of bupivacaine hydrochloride.
- The other ingredients are: sodium chloride, 10% hydrochloric acid (to adjust the pH), water for injections.
What does Bupivacainum hydrochloricum WZF 0.5% look like and what does the packaging contain?
Bupivacainum hydrochloricum WZF 0.5% is a clear and colorless liquid.
Ampoules made of colorless glass, 10 ml - 10 pieces in a cardboard box.
Vials made of colorless glass, 20 ml, closed with a rubber stopper and an aluminum cap - 5 pieces in a cardboard box.
Marketing authorization holder and manufacturer
Marketing authorization holder
Polpharma S.A.
Pelplińska 19, 83-200 Starogard Gdański
tel. +48 22 364 61 01
{vials}
Manufacturer
Polfa Warszawa S.A.
Karolkowa 22/24, 01-207 Warsaw
{ampoules}
Manufacturer
Polpharma S.A.
Pelplińska 19, 83-200 Starogard Gdański
Date of last revision of the leaflet:December 2024
INFORMATION INTENDED ONLY FOR MEDICAL PROFESSIONALS
BUPIVACAINUM HYDROCHLORICUM WZF 0.5% 5 mg/ml, solution for injection
Bupivacaine hydrochloride
Preparation of Bupivacainum hydrochloricum WZF 0.5% for administration and method of administration
- The medicine does not contain preservatives.
- Bupivacainum hydrochloricum WZF 0.5% is intended for infiltration anesthesia, peripheral nerve anesthesia, and epidural anesthesia, as well as for the treatment of acute pain.
- All operations related to dilution and mixing of the product with other medicinal products should be performed under controlled and validated aseptic conditions.
- The medicine can be diluted with 0.9% sodium chloride solution or 5% glucose solution. After dilution with 0.9% sodium chloride solution or 5% glucose solution, chemical and physical stability has been demonstrated for 24 hours at a temperature of 2°C to 8°C (refrigerator). From a microbiological point of view, the diluted product should be used immediately. If it is not used immediately, the user is responsible for the conditions and storage time.
- After the first dose is taken, the unused contents of the vial should be destroyed within 24 hours. After taking a dose of the medicine from the ampoule, the remaining contents of the ampoule should be destroyed immediately.
- Bupivacainum hydrochloricum WZF 0.5% should not be mixed with alkaline solutions, such as bicarbonates, as this may cause precipitation.
- It has been shown that Bupivacainum hydrochloricum WZF 0.5% can be mixed in one syringe with fentanyl, morphine sulfate, or sufentanil. The mixture should be administered immediately. Residues of the solution in the syringe should be discarded.
- This medicine should not be mixed with other medicines, except for those listed above.
- The medicine contains sodium, which should be taken into account in patients with reduced kidney function and in patients controlling their sodium intake (see also the section "Bupivacainum hydrochloricum WZF 0.5% contains sodium").
Instructions for opening the ampoule
Before opening the ampoule, make sure that the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
A colored dot is placed on each ampoule (see Figure 1) as a mark indicating the location of the notch below it.
- To open the ampoule, hold it vertically, in both hands, with the colored dot facing you - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
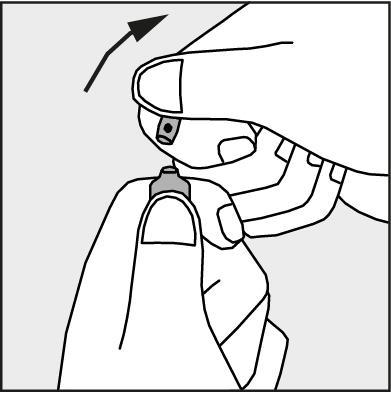
- Press in the direction of the arrow shown in Figure 3. The ampoules are intended for single use only and should be opened immediately before use. The remaining unused product should be destroyed in accordance with applicable regulations.
Figure 1.
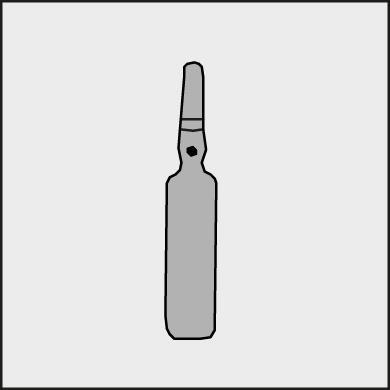
Figure 2.
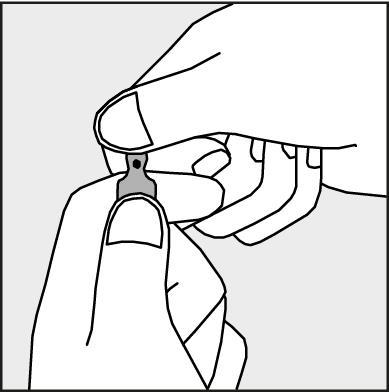
Figure 3.
Warnings and precautions
Particular caution should be exercised when using Bupivacainum hydrochloricum WZF 0.5%:
- in patients with hypovolemia;
- in patients with severe kidney or liver failure;
- in patients after joint injury or surgery;
- in elderly patients in poor general condition.
There have been reports of cardiac arrest and deaths during the use of bupivacaine for epidural or peripheral nerve blockade.
Accidental intravascular administration of bupivacaine should be avoided, as it may cause symptoms of central nervous system toxicity and cardiovascular toxicity.
Epidural anesthesia may cause hypotension and bradycardia.
The risk of these symptoms can be reduced by administering electrolyte or colloid solutions intravenously before anesthesia or by administering vasoconstrictor drugs.
During regional anesthesia, access to equipment and drugs necessary for monitoring and resuscitation of the patient should be ensured.
During the performance of large nerve blocks, the risk of accidental intravascular administration of the product or absorption of the medicine into the systemic circulation increases.
During regional anesthesia of the head and neck, accidental arterial administration of the medicine may occur.
Medicine administered behind the eyeball may rarely penetrate the subarachnoid space of the brain, causing temporary blindness, cardiac arrest, apnea, and seizures.
In the case of eye and retrobulbar anesthesia, there is a risk of persistent eye muscle dysfunction, so the smallest effective concentrations and doses of the product should be used.
Cervical anesthesia may lead to bradycardia or tachycardia in the fetus, so it is necessary to closely monitor the fetal heart rate.
Anesthesia should be performed by a doctor familiar with the technique of anesthesia and trained in the diagnosis and treatment of bupivacaine overdose.
Dosage
Children from 1 to 12 years old
Regional anesthesia procedures in children should be performed by qualified clinicians who are well acquainted with this group of patients and the technique of anesthesia.
The doses listed in the table should be considered as recommended for use in children.
There are individual differences. In children with a large body mass, it is often necessary to reduce the dose and it should be determined based on the patient's body weight.
Factors affecting specific blockade techniques and individual patient requirements are described in anesthesia textbooks. The smallest necessary dose should be used to achieve adequate anesthesia.
The total dose should be determined based on the patient's actual body weight, up to a maximum of 2 mg/kg body weight.
Aspiration should be repeated before and during administration of the dose to avoid intravascular administration. The dose should be administered slowly, in divided doses, especially during epidural administration in the lumbar and thoracic segments, while constantly and carefully monitoring the patient's vital functions.
Infiltration anesthesia around the tonsils was performed in children over 2 years of age using bupivacaine at a concentration of 2.5 mg/ml, administered in a dose of 7.5 to 12.5 mg per tonsil.
Ilioinguinal-iliohypogastric blocks were performed in children from 1 year of age or older using bupivacaine at a concentration of 2.5 mg/ml, administered in a dose of 0.1-0.5 ml/kg body weight, which corresponds to 0.25-1.25 mg/kg body weight. Children from 5 years of age or older received bupivacaine at a concentration of 5 mg/ml, administered in a dose of 1.25-2 mg/kg body weight.
To perform a penile block, bupivacaine at a concentration of 5 mg/ml was used, administered in a total dose of 0.2-0.5 ml/kg body weight, which corresponds to 1-2.5 mg/kg body weight.
The safety and efficacy of Bupivacainum hydrochloricum WZF 0.5% have not been established in children under 1 year of age. Available data are limited.
The safety and efficacy of intermittent injections (boluses) administered epidurally or continuous infusion have not been established. Available data are limited.
Adults and adolescents from 12 years old
The following table shows the recommended dosages of the medicine for the most commonly used anesthesia techniques. The individual dose should be calculated based on the doctor's experience and the patient's general condition.
In the case of prolonged anesthesia using the technique of continuous anesthesia or repeated doses, the possibility of achieving toxic serum concentrations or local nerve damage should be considered.
The medicine is often used in epidural anesthesia for pain treatment in combination with an opioid drug. The total dose should not exceed 400 mg/24 hours.
If bupivacaine is used simultaneously in other anesthesia techniques, the total dose of bupivacaine should not exceed 150 mg.
Post-marketing observations have reported cases of chondrolysis in patients receiving postoperative continuous intra-articular infusion of local anesthetics.
The doses listed in the table are considered sufficient to induce anesthesia in an adult patient. The onset and duration of action may vary between individual patients. The values listed in the table are usually the required dose range. Various factors that may affect specific anesthesia techniques and individual patient requirements should be taken into account.
Unnecessary use of large doses of local anesthetics should be avoided. To achieve complete blockade of all nerve fibers in large nerves, higher concentrations of the medicine are required. To achieve complete blockade of smaller nerves or less intense anesthesia (e.g., relief of labor pain), smaller concentrations are recommended.
The volume of the administered medicine determines the size of the anesthetized area.
Aspiration should be performed carefully before and during administration of the medicine to avoid intravascular administration. The main dose of bupivacaine should be administered slowly, at a rate of 25 mg to 50 mg/min, or the medicine should be administered in divided doses. At the same time, vital functions should be carefully monitored and verbal contact with the patient should be maintained.
Before administering the medicine into the epidural space, a test dose of 3 to 5 ml of bupivacaine solution with adrenaline is recommended. Accidental intravascular administration of the medicine can be recognized based on transient acceleration of heart rate, and accidental administration into the subarachnoid space can be recognized based on the occurrence of blockade symptoms. In case of acute symptoms of toxicity, the administration of the medicine should be stopped immediately.
Procedure in case of acute toxicity symptoms
In case of acute toxicity symptoms, the administration of the medicine should be stopped immediately.
In case of hypotension and bradycardia, 5 to 10 mg of ephedrine should be administered intravenously. The dose can be repeated after 2-3 minutes if necessary. The dose of ephedrine used in children depends on the age and body weight. In case of cardiac arrest, resuscitation may be necessary for a long time.
In case of cardiac arrest, cardiopulmonary resuscitation should be started immediately. It is very important to ensure proper oxygenation of the blood, ventilation, and circulation support.
Treatment of patients who have experienced seizures aims to maintain ventilation and ensure adequate oxygenation, stop the seizure, and maintain circulation. Oxygen should be administered if necessary, assisted or controlled ventilation should be used (oxygen mask, Ambu bag, or endotracheal intubation). If the seizure does not stop within 15 to 20 seconds, an anticonvulsant drug should be administered intravenously. Thiopental administered intravenously in a dose of 1-3 mg/kg body weight causes rapid cessation of seizures. Diazepam can also be administered intravenously in a dose of 0.1 mg/kg body weight, but seizures cease much more slowly. Prolonged seizures can cause respiratory and oxygenation disorders in the patient. In such a case, a muscle relaxant, such as succinylcholine, can be administered in a dose of 1 mg/kg body weight, the patient can be intubated, and ventilation can be performed.
- Country of registration
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
Online doctors for Bupivacainum hidrohloricum Vzf 0,5%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Bupivacainum hidrohloricum Vzf 0,5% – subject to medical assessment and local rules.














