

Briglau Pph

Ask a doctor about a prescription for Briglau Pph

How to use Briglau Pph
Package Leaflet: Information for the Patient
Briglau PPH, 2 mg/ml, Eye Drops, Solution
Brimonidine Tartrate
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Briglau PPH and what is it used for
- 2. Important information before using Briglau PPH
- 3. How to use Briglau PPH
- 4. Possible side effects
- 5. How to store Briglau PPH
- 6. Contents of the pack and other information
1. What is Briglau PPH and what is it used for
Briglau PPH is a medicine that lowers intraocular pressure (pressure in the eye).
Briglau PPH is used alone or in combination with other medicines to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension (high fluid pressure in the eye).
The active substance in Briglau PPH is brimonidine tartrate, which reduces pressure in the eye.
2. Important information before using Briglau PPH
When not to use Briglau PPH
- if you are allergic to brimonidine tartrate or any of the other ingredients of this medicine (listed in section 6).
- if you are taking a monoamine oxidase inhibitor (MAOI) or certain antidepressants (such as tricyclic antidepressants or mianserin). You should ask your doctor if you can use Briglau PPH if you are taking an antidepressant.
- if you are breastfeeding.
- in newborns and infants (from birth to 2 years).
Warnings and precautions
Before starting treatment with Briglau PPH, you should discuss it with your doctor if:
- you have depression, reduced mental ability, cerebral circulatory disorders, heart disease, circulatory disorders in the limbs, or blood pressure disorders.
- you have or have had liver or kidney function disorders in the past.
Children
Briglau PPH is not recommended for use in children under 12 years of age. If the medicine has been prescribed for a child under 12 years of age, you should talk to your doctor before using it.
Briglau PPH and other medicines
You should tell your doctor or pharmacist about all medicines you are taking now or have taken recently, as well as about medicines you plan to take.
You should tell your doctor about taking the following medicines, as they may affect treatment with Briglau PPH:
- analgesics, sedatives, opioids, barbiturates, or regularly consumed alcohol,
- anesthetics,
- medicines used to treat heart diseases or lower blood pressure,
- metabolism-affecting medicines such as chlorpromazine, methylphenidate, and reserpine,
- medicines that act on the same receptor as Briglau PPH, such as isoprenaline and prazosin,
- monoamine oxidase inhibitors and other antidepressants,
- medicines used for other diseases, even if not related to eye disease. You should also tell your doctor about any changes in the dosage of medicines you are currently taking.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, you should consult your doctor before using this medicine.
Do not use Briglau PPH during breastfeeding.
Driving and using machines
Briglau PPH may cause blurred vision or visual disturbances, especially at night or in low light conditions.
In some patients, Briglau PPH may also cause drowsiness or fatigue.
If you experience these symptoms, you should not drive or operate machinery until they have resolved.
Briglau PPH contains benzalkonium chloride
The medicine contains 0.05 mg of benzalkonium chloride per milliliter of solution.
Benzalkonium chloride may be absorbed by soft contact lenses and change their color. You should remove contact lenses before instillation and wait at least 15 minutes before reinserting them.
Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or corneal disorders (the transparent layer on the front of the eye). If you experience any abnormal sensations in the eye, stinging, or pain in the eye after using the medicine, you should contact your doctor.
3. How to use Briglau PPH
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist.
Adults and adolescents over 12 years of age
The recommended dose is one drop into the affected eye(s) twice a day, approximately every 12 hours.
Children under 12 years of age
Briglau PPH should not be used in children under 2 years of age.
It is not recommended to use Briglau PPH in children between 2 and 12 years of age.
Method of administration
Briglau PPH is intended for use in the eye only. Do not take the medicine orally.
Before instillation, always wash your hands. Use the number of drops as directed by your doctor. If you are taking more than one eye medicine, wait at least 5-15 minutes between medications.
Instill the medicine as follows:
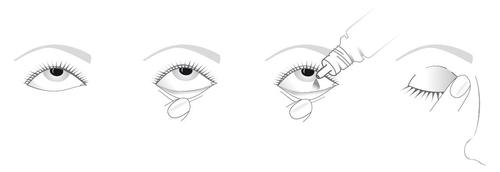
- 1. Tilt your head back and look up at the ceiling.
- 2. Gently pull down the lower eyelid to create a small pocket.
- 3. Hold the bottle upside down and squeeze it to release a drop into the eye.
- 4. Immediately after administering each drop, close your eye and press the inner corner of the eye (near the nose) with your finger for 1 minute.
Avoid touching the dropper tip to the eye or anything else. Replace the cap after use. If you wear soft contact lenses, remove them before using the medicine and wait 15 minutes before reinserting them after instillation. The preservative in the drops can discolor soft contact lenses.
Using more than the recommended dose of Briglau PPH
Adults
In adults who have been given more drops than recommended, the reported side effects were those currently known to occur with Briglau PPH.
In adults who have accidentally taken Briglau PPH orally, a drop in blood pressure has been reported, followed by an increase in blood pressure in some patients.
Children
Severe side effects have been reported in children who have accidentally taken Briglau PPH orally. The following symptoms have been observed: drowsiness, decreased muscle tone, decreased body temperature, pallor, and breathing difficulties. If you experience any of these symptoms, you should contact your doctor immediately.
Adults and children
If someone has accidentally ingested the medicine, you should contact your doctor immediately.
Missing a dose of Briglau PPH
If you miss a dose, use it as soon as possible. However, if it is almost time for the next dose, skip the missed dose and continue with your regular schedule.
Stopping treatment with Briglau PPH
To be effective, the medicine should be used every day. Do not stop or discontinue treatment without consulting your doctor first.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Briglau PPH can cause side effects, although not everybody gets them.
Side effects may occur with a certain frequency, defined as follows:
After using Briglau PPH, the following eye-related side effectsmay occur:
Very common:
Eye irritation (eye redness, burning, stinging, feeling of a foreign body, itching, lumps or white spots on the conjunctiva), blurred vision, allergic reactions of the eye.
Common:
Changes on the surface of the eye, eyelid inflammation, conjunctivitis, visual disturbances, swelling of the eyelids or conjunctiva, increased sensitivity to light, irritation, redness, pain, feeling of dryness in the eyes, superficial damage or discoloration on the surface of the eye, tearing or conjunctival blanching.
Very rare:
Iritis, miosis.
Frequency not known:
Itching of the eyelids.
The following side effects related to other parts of the bodymay also occur:
Very common:
Headache, dry mouth, and feeling of tiredness and/or drowsiness.
Common:
Dizziness, symptoms similar to a cold, stomach and/or intestinal pain, taste disturbances, and weakness.
Uncommon:
Depression, palpitations or irregular heartbeat, dryness of the nasal mucosa, generalized allergic reactions.
Rare:
Dyspnea.
Very rare:
Insomnia, fainting, high or low blood pressure.
Frequency not known:
Skin reactions including redness, facial swelling, itching, rash, and vasodilation.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocides of the Office for Registration of Medicinal Products, Medical Devices, and Biocides
| Very common | occur in more than 1 in 10 patients |
| Common | occur in less than 1 in 10 patients |
| Uncommon | occur in less than 1 in 100 patients |
| Rare | occur in less than 1 in 1,000 patients |
| Very rare | occur in less than 1 in 10,000 patients |
| Not known | frequency cannot be estimated from available data |
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Briglau PPH
Do not store above 25°C.
Keep the medicine out of the sight and reach of children.
Do not use the drops if the protective ring is damaged before first use.
Do not use this medicine after the expiry date stated on the bottle and carton after EXP.
The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
The bottle should be discarded 28 days after first opening, even if the solution is still present.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Briglau PPH contains
- The active substance is brimonidine tartrate. Each milliliter of solution contains 2 mg of brimonidine tartrate, equivalent to 1.3 mg of brimonidine.
- The other ingredients are: benzalkonium chloride (as a preservative), polyvinyl alcohol, sodium chloride, sodium citrate, citric acid monohydrate, hydrochloric acid or sodium hydroxide (to adjust pH), water for injections.
What Briglau PPH looks like and contents of the pack
Briglau PPH is a clear, slightly yellowish solution in a plastic bottle.
The bottle contains 5 ml of solution.
Pack sizes: 1 x 5 ml, 3 x 5 ml.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Polpharma S.A.
Pelplińska 19
83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Briglau PphDosage form: Drops, 2 mg/mlActive substance: brimonidineManufacturer: Allergan Pharmaceuticals IrelandPrescription requiredDosage form: Drops, 2 mg/mlActive substance: brimonidinePrescription requiredDosage form: Drops, 2 mg/mlActive substance: brimonidineManufacturer: Rafarm S.A.Prescription required
Alternatives to Briglau Pph in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Briglau Pph in Ukraine
Alternative to Briglau Pph in Spain
Online doctors for Briglau Pph
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Briglau Pph – subject to medical assessment and local rules.














