How to use Betadine Vag
Package Leaflet: Information for the User
Please read carefully the contents of the leaflet before using the medicine, as it contains
important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor or pharmacist.
- You should keep this leaflet, so you can read it again if you need to.
- If you need advice or further information, you should speak to your pharmacist.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. See section 4.
- If after 7 days there is no improvement or you feel worse, you should contact your doctor.
Table of Contents of the Leaflet
- 1. What is Betadine VAG and what is it used for
- 2. Important information before using Betadine VAG
- 3. How to use Betadine VAG
- 4. Possible side effects
- 5. How to store Betadine VAG
- 6. Contents of the pack and other information
1. What is Betadine VAG and what is it used for
Betadine VAG is intended for vaginal use only. Betadine VAG contains povidone-iodine as the active substance. Povidone-iodine is a complex of iodine with povidone - after vaginal application, it releases iodine. Iodine acts on Gram-positive and Gram-negative bacteria, viruses, fungi, and some protozoa.
Indications for use
Betadine VAG is indicated for the treatment of vaginal infections.
Betadine VAG is used in adult women and girls after puberty.
If after 7 days there is no improvement or you feel worse, you should contact your doctor.
2. Important information before using Betadine VAG
When not to use Betadine VAG
- if you are allergic to povidone-iodine or any of the other ingredients of this medicine (listed in section 6),
- if your thyroid gland does not work properly (you have been diagnosed with hyperthyroidism or other symptomatic thyroid disorders - see section "Warnings and precautions"),
- before, during, and after treatment with radioactive iodine and scintigraphy,
- in herpetic-like skin rash (so-called herpetic dermatitis of Duhring),
- in girls before puberty (before the first menstrual period),
- in pregnant and breastfeeding women,
- if you are using products containing mercury, as concomitant use of Betadine VAG with products containing mercury may cause skin damage.
Using Betadine VAG should be discussed with your doctor or pharmacist if you experience any of the following symptoms:
- Irregular vaginal bleeding
- Abnormal vaginal bleeding or discharge
- Ulcers, blisters, or sores on the vagina or vulva
- Pain or difficulty urinating
If any of the following symptoms occur in the case of vaginal infections, you should contact your doctor before using Betadine VAG:
- Fever or chills
Warnings and precautions
Before starting to use Betadine VAG, you should discuss it with your doctor or pharmacist.
- Patients with pre-existing kidney failure should consult their doctor before using Betadine VAG.
- Regular use of Betadine VAG globules should be avoided in case of concomitant use of lithium-containing medications.
- Prolonged use of povidone-iodine-containing medications may cause skin irritation, and in rare cases, severe skin reactions. If irritation or hypersensitivity occurs, you should stop using the medicine and contact your doctor immediately (see section 4. Possible side effects).
- The use of povidone-iodine (Betadine VAG) may lead to temporary skin discoloration at the application site, which is due to the color of the medicine itself.
- The medicine may cause false-positive results in some laboratory tests, such as detection of occult blood in stool or urine and detection of glucose in urine.
- If after 7 days there is no improvement, you should contact your doctor.
- If within 6 months more than 2 vaginal infections occur, you should contact your doctor.
Children and adolescents
Betadine VAG is contraindicated for use in girls before puberty.
Betadine VAG and other medicines
You should tell your doctor or pharmacist about all medicines you are taking now or have taken recently (including those without a prescription) and about medicines you plan to take.
Products containing mercury (see section "When not to use Betadine VAG"), silver, hydrogen peroxide, and taurolidine may interact with povidone-iodine and should not be used concomitantly.
Concomitant use of Betadine VAG with wound care products containing enzymatic ingredients leads to reduced efficacy of both medicines, so they should not be used concomitantly.
Betadine VAG should not be used concomitantly with reducing agents or alkali metal salts.
Medicines containing povidone-iodine (Betadine VAG) used before or after antiseptic products containing octenidine may cause transient dark discoloration in affected areas, so they should not be used concomitantly.
During the use of povidone-iodine, there may be a decrease in iodine uptake by the thyroid gland, which may interfere with the results of some diagnostic tests of thyroid function [(e.g., thyroid scintigraphy, PBI (protein-bound iodine) measurement, radioactive iodine diagnostics] and may prevent planned thyroid treatment with iodine (radioiodine therapy). To obtain objective scintigraphy results, it is recommended to ensure a sufficiently long period (at least 4 weeks) after the end of treatment with Betadine VAG.
Betadine VAG may cause false-positive results in some diagnostic tests (such as detection of blood in stool or glucose in urine). You should inform your doctor about the use of Betadine VAG before laboratory tests.
Pregnancy, breastfeeding, and fertility
Pregnancy
Use of povidone-iodine-containing globules in pregnant women is contraindicated, as absorbed iodine may cross the placental barrier.
Breastfeeding
Use of Betadine VAG is contraindicated in breastfeeding women, as absorbed iodine may pass into breast milk (see section 5.2).
Fertility
There are limited data on the effect of povidone-iodine on human fertility. Betadine VAG globules have spermicidal properties. Therefore, their use is not recommended in women planning to become pregnant.
If you think you may be pregnant or plan to have a baby, you should consult your doctor or pharmacist before using this medicine.
Driving and using machines
The medicine has no effect on the ability to drive and use machines.
3. How to use Betadine VAG
Betadine VAG should always be used exactly as described in the patient leaflet or as directed by your doctor or pharmacist. In case of doubt, you should consult your doctor or pharmacist.
Vaginal use.
Do not heat before application.
Betadine VAG is intended for use in adult women and girls after puberty.
Adults
It is recommended to use 1 globule vaginally once a day for 7 days. If there is no improvement, you should consult your doctor.
It is recommended to moisten the globule before use and then insert it deep into the vagina in the evening before going to bed.
Application of the globule:
- You should separate one globule from the strip by tearing along the perforation at the "V" cut.
- Hold the plastic tabs with both hands and pull them apart to remove the globule from the packaging.
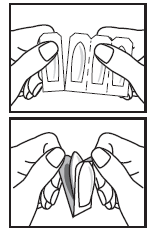
It is also recommended to use sanitary pads during treatment.
It is essential to moisten the globule before insertion to ensure maximum dissolution of the active substance and avoid local irritation. Discontinuation of treatment is not necessary even after menstrual bleeding occurs.
Use in children and adolescents
Betadine VAG is contraindicated for use in girls before puberty.
Using more than the recommended dose of Betadine VAG
Betadine VAG is intended for vaginal use only.
When used according to the instructions, there is no risk of overdose.
In case of local overdose, it is recommended to remove the inserted globule.
If you use more than the recommended dose of Betadine VAG or accidentally swallow a globule, you should contact your doctor immediately.
Excess iodine in the body may cause goiter, thyroid dysfunction.
Symptoms of acute iodine poisoning are:
- renal failure and lack of urine production (anuria),
- circulatory failure (shock),
- breathing difficulties (pulmonary edema),
- metabolic disorders
If you notice any of the following symptoms of systemic toxicity, you should seek medical help immediately:
- renal dysfunction (including anuria),
- rapid heartbeat (tachycardia),
- low blood pressure (hypotension),
- circulatory collapse,
- breathing difficulties (laryngeal edema leading to asphyxia or pulmonary edema),
- seizures,
- fever,
- metabolic acidosis,
- hyperthyroidism or hypothyroidism may also develop (see section 4. "Possible side effects", frequency "very rare" and "frequency not known").
Missing a dose of Betadine VAG
You should continue treatment as before and use the medicine as soon as possible.
You should not use a double dose to make up for a missed dose.
Stopping the use of Betadine VAG
To prevent the recurrence of the disease and ensure complete cure of the infection, you should not stop treatment before the end of 7 days.
If you have any further doubts about the use of this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In case of the following side effects, you should stop using Betadine VAG and contact your doctor immediately
.
Rare side effects (occurring in less than 1 in 1000 patients):
- Hypersensitivity reactions.
Very rare side effects (occurring in less than 1 in 10,000 patients):
- Anaphylactic reaction (a severe allergic reaction causing difficulty breathing, dizziness, and low blood pressure).
- Angioedema (a severe allergic reaction causing swelling of the face or throat).
The following may also occur:
Rare (occurring in less than 1 in 1000 patients):
Skin irritation (so-called contact dermatitis with symptoms such as redness, small blisters, and itching).
Very rare (occurring in less than 1 in 10,000 patients):
Hyperthyroidism (excessive thyroid activity causing increased appetite, weight loss, sweating, faster heartbeat, or restlessness) in patients with a history of thyroid disease.
Side effects of unknown frequency(based on available data, the frequency cannot be estimated):
- After prolonged or more intense use of povidone-iodine, hypothyroidism (insufficient thyroid activity causing fatigue, weight gain, slower heartbeat) may occur.
- Renal dysfunction, acute renal failure;
- Electrolyte disturbances (imbalance of fluids and salts in the blood),
- Metabolic acidosis (a condition of increased blood acidity that may cause rapid breathing, disorientation, or lethargy),
- Abnormal blood osmolality (referring to the degree of blood concentration) may occur after taking very large amounts of povidone-iodine.
- Transient skin discoloration at the application site.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Pharmacovigilance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Betadine VAG
Store in the refrigerator (2-8°C).
The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the carton after: Expiry date (EXP). The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Betadine VAG contains
The active substance of the medicine is povidone-iodine.
Each globule contains 200 mg of povidone-iodine.
The other ingredient of the medicine is macrogol 1000.
What Betadine VAG looks like and contents of the pack
The medicine is in the form of globules. Each globule is uniform, brown-red in color, torpedo-shaped, approximately 33 mm long, and approximately 12 mm in diameter. The pack contains 7 globules (1 blister pack of PVC/PE), placed in a cardboard box.
Marketing authorization holder
Egis Pharmaceuticals PLC.
1106 Budapest, Keresztúri út 30-38.
Hungary
Manufacturer
Egis Pharmaceuticals PLC Lacta Factory
Mátyás király u. 65
9900 Körmend
Hungary
To obtain more detailed information about this medicine, you should contact the local representative of the marketing authorization holder:
EGIS Polska Sp. z o.o.
ul. Komitetu Obrony Robotników 45D
02-146 Warsaw
Phone: +48 22 417 92 00
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterEgis Pharmaceuticals Ltd.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Betadine VagDosage form: Suppositories, 200 mgActive substance: povidone-iodinePrescription not requiredDosage form: Suppositories, 90 mgActive substance: policresulenManufacturer: Takeda GmbHPrescription not requiredDosage form: Cream, 10 mg/gActive substance: ciclopiroxManufacturer: Doppel Farmaceutici S.r.l.Prescription not required
Alternatives to Betadine Vag in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Betadine Vag in Ukraine
Alternative to Betadine Vag in Espanha
Online doctors for Betadine Vag
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Betadine Vag – subject to medical assessment and local rules.