

Bcg Shhepionka Aivaccines

Ask a doctor about a prescription for Bcg Shhepionka Aivaccines

How to use Bcg Shhepionka Aivaccines
Package Leaflet: Information for the User
BCG Vaccine AJVaccines
Powder and solvent for solution for injection.
Mycobacterium bovisBCG (Bacillus Calmette-Guérin), Danish strain 1331, live attenuated.
Read the package leaflet carefully before using the vaccine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed for a specific person. Do not pass it on to others.
- If the patient experiences any side effects, including any not listed in the leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Package Leaflet
- 1. What is BCG Vaccine AJVaccines and what is it used for
- 2. Important information before using BCG Vaccine AJVaccines
- 3. How to use BCG Vaccine AJVaccines
- 4. Possible side effects
- 5. How to store BCG Vaccine AJVaccines
- 6. Contents of the pack and other information
1. What is BCG Vaccine AJVaccines and what is it used for
BCG Vaccine AJVaccines contains live attenuated Mycobacterium bovisBCG and is a vaccine used for protection against tuberculosis (TB).
2. Important information before using BCG Vaccine AJVaccines
Vaccinations with BCG Vaccine AJVaccines should not be performed:
- if the patient is allergic to Mycobacterium bovisor any of the other components of this vaccine (listed in section 6),
- if the patient has a severe acute illness with fever or generalized skin infection - in these cases, vaccination should be postponed,
- if the patient has impaired immune response to infections or immune system disorders,
- if the patient is undergoing treatment, e.g., with corticosteroids or treatment that impairs the immune system (including radiotherapy),
- if the patient has been exposed to immunosuppressive agents in utero or through breastfeeding (e.g., TNF-α antagonists),
- if the patient has any malignant disease (e.g., lymphoma, leukemia, or Hodgkin's disease),
- if the patient's immune system status is unknown,
- if the patient has HIV infection,
- if the patient is taking anti-tuberculosis medication (TB).
Warnings and precautions
You should talk to your doctor, pharmacist, or nurse before vaccination with BCG Vaccine AJVaccines. The doctor or nurse will take special care with regard to vaccination with BCG Vaccine AJVaccines
- if the patient has eczema. BCG Vaccine AJVaccines may be given, but in an area free from lesions.
- if the patient has a positive tuberculin test result. Administration of the vaccine to individuals with a positive tuberculin test result does not provide any benefit, but may cause a severe local reaction.
BCG Vaccine AJVaccines and other medicines
Tell your doctor or pharmacist about all medicines the patient is taking, has recently taken, or might take. Other vaccines can be given at the same time as BCG Vaccine AJVaccines, but in a different location.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before using BCG Vaccine AJVaccines. Vaccination is not recommended during pregnancy and breastfeeding, although no harmful effects of BCG Vaccine AJVaccines on the unborn child or breastfed child have been reported.
Driving and using machines
BCG Vaccine AJVaccines has no influence on the ability to drive and use machines.
BCG Vaccine AJVaccines contains potassium and sodium
BCG Vaccine AJVaccines contains less than 1 mmol of potassium (39 mg) per dose, so it can be considered potassium-free. BCG Vaccine AJVaccines contains less than 1 mmol of sodium (23 mg) per dose, so it can be considered sodium-free.
3. How to use BCG Vaccine AJVaccines
The doctor or nurse will give the vaccine by injection into the superficial layer of the skin. The recommended dose is: Infants under 12 months: 0.05 ml Adults and children over 12 months: 0.1 ml To facilitate healing, the injection site should be left uncovered. Reactions that may occur after vaccination:
- a small swelling, redness, and tenderness at the injection site until a skin lesion forms
- on the skin
- after a few weeks, the skin lesion turns into a small ulcer
- after a few months, the ulcer heals, leaving only a small, flat scar
- a small swelling of the lymph nodes in the armpit may occur
These are common reactions to the vaccine. If you have any further questions about using this vaccine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, BCG Vaccine AJVaccines can cause side effects, although not everybody gets them. Severe allergic reactions (such as redness of the face and neck, swelling of the face, throat, and neck, skin rash, breathing problems, collapse) may occur rarely (less than 1 in 1,000). If any of the above reactions occur, contact your doctor immediately.
Other side effects include:
Uncommon side effects (may affect up to 1 in 100 people)
- fever
- swelling of the lymph nodes in the armpit with a diameter greater than 1 cm
- lymph node inflammation, sometimes with discharging ulcer and pus
- discharging ulcer at the injection site
- headache
Rare side effects (may affect up to 1 in 1,000 people)
- abscess at the injection site
- bacterial infection from the vaccine, which can lead to generalized infection, including bone infection
Fainting, seizures, and convulsions have been observed in patients vaccinated. In premature infants (born at 28 weeks of gestation or earlier), longer pauses between breaths may occur within 2-3 days after vaccination.
Reporting side effects
If you experience any side effects, including any not listed in the leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222, Warsaw, Tel. +48 22 492 13 01, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store BCG Vaccine AJVaccines
Store the vaccine out of sight and reach of children. Store in a refrigerator (2 – 8°C). Store in the original package to protect from light. Do not freeze. Do not use after the expiry date stated on the carton after “Exp”. The expiry date refers to the last day of the month. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What BCG Vaccine AJVaccines contains
The active substance is a freeze-dried powder containing live, attenuated Mycobacterium bovisBCG (Bacillus Calmette-Guérin), Danish strain 1331. 1 ml of reconstituted vaccine contains 2 to 8 million bacteria. The other ingredients are: sodium glutamate, magnesium sulfate heptahydrate, dipotassium phosphate, asparagine monohydrate, iron (III) ammonium citrate, glycerol 85%, citric acid monohydrate, water for injections.
What BCG Vaccine AJVaccines looks like and contents of the pack
BCG Vaccine AJVaccines consists of a powder and solvent for solution for injection (2-8 x 10 bacteria/0.1 ml or 1-4 x 10 bacteria/0.05 ml). The pack sizes are: powder: 1, 5, or 10 vials, and a pack containing 1 vial with one syringe and two needles (one long for solvent withdrawal, one short for intradermal injection). The powder in the vial is white and crystalline. The powder may be difficult to see due to the small amount in the vial. The solvent in the clear vial is a colorless solution without any visible particles. The reconstituted vaccine should be a homogeneous, slightly opalescent, and colorless suspension. The pack sizes for the solvent are: 1, 5, and 10 vials, and a pack containing 1 vial in a single-dose injection set. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
AJ Vaccines A/S 5, Artillerivej DK-2300 Copenhagen S Denmark tel: +45 7229 7000 e-mail: [email protected]
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
DK: BCG Vaccine “AJ Vaccines” EL, FI: BCG Vaccine AJVaccines FR: VACCIN BCG AJVaccines NO: BCG-vaksine AJVaccines PL: BCG Szczepionka AJVaccines SE: BCG-vaccin AJVaccines UK: BCG Vaccine AJV Date of revision of the leaflet:04/2024
INFORMATION INTENDED FOR MEDICAL PERSONNEL ONLY
Special warnings and precautions for use
The vaccine should be administered precisely intradermally. BCG Vaccine AJVaccines should be administered by medical personnel trained in the administration of intradermal injections. Incorrect administration, e.g., subcutaneously or intramuscularly, increases the risk of lymph node inflammation and abscess formation. Individuals with a positive tuberculin test result should not be vaccinated, as this may cause a severe local reaction. Although anaphylactic reactions are rare, the necessary treatment should always be available during vaccination. If possible, the patient should be kept under observation for about 15-20 minutes after vaccination in case of allergic reactions. BCG Vaccine AJVaccines can be given at the same time as other inactivated or live vaccines, including combined measles, mumps, and rubella vaccine. If other vaccines are not given at the same time, a minimum interval of 4 weeks should be observed before administering another live vaccine. For at least 3 months, no other vaccine should be injected into the arm that received BCG Vaccine AJVaccines.
Preparation for administration
The rubber stopper should not be wiped with any antiseptic or detergent. If the rubber stopper of the vial is wiped with alcohol, wait until it has evaporated before inserting the needle. Using a syringe with a long needle, draw up the solvent (the volume indicated on the label) and transfer it to the vial with the powder. Do not use other solvents, as they may destroy the vaccine. Gently turn the vial upside down several times to ensure complete dissolution of the freeze-dried BCG Vaccine AJVaccines. Do not shake the vial. Before drawing up each subsequent dose, gently turn the vial with the suspension. The reconstituted vaccine should be a homogeneous, slightly opalescent, and colorless suspension. After reconstitution, the vaccine should be used within 4 hours.
Method of administration:
BCG Vaccine AJVaccines should be administered by medical personnel trained in the administration of intradermal injections. The injection site should be clean and dry. If the skin is cleaned with alcohol or similar antiseptics before vaccination, wait until they have evaporated completely. The vaccine must be injected precisely intradermally into the arm, above the distal insertion of the deltoid muscle to the humerus (in the upper third of the arm), as follows:
- Hold the skin between the thumb and index finger.
- Insert the needle almost parallel to the skin surface, with the bevel facing upwards, slowly to a depth of about 2 mm into the superficial skin layers. The needle should be visible through the skin during insertion.
- Administer the injection slowly.
- A raised white blister at the injection site is a sign of a correctly performed injection.
- The injection site should be left uncovered to facilitate healing.
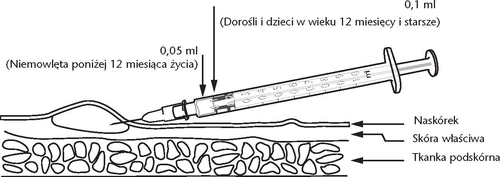
The reconstituted vaccine should be administered using a 1 ml syringe with a graduated scale to one hundredth of a milliliter (0.01 ml) with a short needle with a cut tip (25G/0.50 mm or 26 G/0.45 mm). The vaccine should not be administered through jet injectors or devices for multiple punctures.
Overdose or incorrect administration
Overdose increases the risk of suppurative lymphadenitis and may lead to the formation of an excessively large scar. Overdose increases the risk of undesirable complications. Administration of the vaccine too deeply increases the risk of ulceration, lymph node inflammation, and abscess formation.
Treatment of complications after BCG Vaccine AJVaccines
Consult an expert to determine the appropriate treatment for general or persistent local infections following BCG Vaccine AJVaccines. Sensitivity of the BCG strain to antibiotics: The table below shows the minimum inhibitory concentrations (MIC) for selected anti-tuberculosis drugs against BCG Danish strain 1331 [determined using Bactec 460]. The MIC for isoniazid is 0.4 mg/l. There is no consensus on whether Mycobacterium bovisBCG should be classified as susceptible, moderately susceptible, or resistant to isoniazid when the MIC is 0.4 mg/l. However, based on the criteria adopted for Mycobacterium tuberculosis, the strain may be considered moderately susceptible.
| Drug | Minimum Inhibitory Concentration (MIC) |
| Isoniazid | 0.4 mg/l |
| Streptomycin | 2.0 mg/l |
| Rifampicin | 2.0 mg/l |
| Etambutol | 2.5 mg/l |
BCG Danish strain 1331 is resistant to pyrazinamide.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAJ Vaccines A/S
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Bcg Shhepionka AivaccinesDosage form: Powder, 0.5 mg (from 1.5 million to 6 million live BCG bacilli/ml); 10-dose vaccine, 1 dose (0.1 ml)Active substance: tuberculosis, live attenuatedPrescription requiredDosage form: Powder, 10 mcg Haemophilus influenzae type b polysaccharide conjugated with 18-30 mcg tetanus toxoid/0.5 ml; 1 dose (0.5 ml)Active substance: haemophilus influenzae B, purified antigen conjugatedManufacturer: Sanofi PasteurPrescription requiredDosage form: Suspension, 1 dose (0.5 ml)Active substance: pertussis, purified antigen, combinations with toxoidsPrescription required
Alternatives to Bcg Shhepionka Aivaccines in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Bcg Shhepionka Aivaccines in Ukraine
Alternative to Bcg Shhepionka Aivaccines in Spain
Online doctors for Bcg Shhepionka Aivaccines
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Bcg Shhepionka Aivaccines – subject to medical assessment and local rules.














