
Augmentin Mff
Ask a doctor about a prescription for Augmentin Mff

How to use Augmentin Mff
Leaflet accompanying the packaging: information for the user
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Augmentin MFF(Augmentin DUO)
(400 mg + 57 mg)/5 ml, powder for oral suspension
Amoxicillin + Clavulanic acid
Augmentin MFF and Augmentin DUO are different trade names for the same medicine.
You should carefully read the contents of the leaflet before starting to use this medicine in a child, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor, pharmacist, or nurse.
- This medicine is usually prescribed for children. Do not give it to others. The medicine may harm another person, even if their symptoms are the same as those of the child.
- If the child experiences any side effects, including any side effects not listed in this leaflet, you should tell the doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Augmentin MFF and what is it used for
- 2. Important information before taking Augmentin MFF
- 3. How to take Augmentin MFF
- 4. Possible side effects
- 5. How to store Augmentin MFF
- 6. Contents of the packaging and other information
1. What is Augmentin MFF and what is it used for
Augmentin MFF is an antibiotic that works by killing bacteria that cause infections. Augmentin MFF contains two different medicines: amoxicillin and clavulanic acid.
Amoxicillin belongs to a group of medicines called "penicillins", which may sometimes be inactivated (deactivated). The second active ingredient (clavulanic acid) counteracts this deactivation.
Augmentin MFF is used in infants and children to treat the following infections:
- middle ear and sinus infections
- respiratory tract infections
- urinary tract infections
- skin and soft tissue infections, including dental infections
- bone and joint infections.
2. Important information before taking Augmentin MFF
When not to give Augmentin MFF to a child
- If the patient has been found to be allergic to amoxicillin, clavulanic acid, penicillin, or any of the other ingredients of this medicine (listed in section 6).
- If the patient has ever had severe allergic reactions to any other antibiotic. These may include skin rash or swelling of the face or throat.
- If the patient has ever had liver function disorders or jaundice (yellowing of the skin) associated with the use of an antibiotic.
- If the above circumstances apply to the child being treated, do not give them Augmentin MFF.If in doubt before starting treatment with Augmentin MFF, consult the doctor or pharmacist.
Warnings and precautions
Before starting to give Augmentin MFF to a child, consult the doctor, pharmacist, or nurse if:
- the child has infectious mononucleosis
- the child is being treated for liver or kidney disease
- the child has irregular urination. If in doubt whether the above circumstances apply to the child being treated, consult the doctor or pharmacist before starting treatment with Augmentin MFF.
In some cases, the doctor may test what type of bacteria caused the infection in the patient. Depending on the results, the child may receive Augmentin MFF in a different dose or a different medicine.
Conditions to watch out for
Taking Augmentin MFF may worsen the course of certain diseases or cause severe side effects, including allergic reactions, seizures, and colitis. You should pay attention to whether the child being treated experiences certain symptoms during treatment with Augmentin MFF, in order to reduce the risk of any problems. See "Conditions to watch out for" in section 4.
Blood and urine tests
If the child is to have blood tests (such as red blood cell tests or liver function tests) or urine tests (for glucose), you should inform the doctor or nurse that the child is taking Augmentin MFF. Augmentin MFF may affect the results of these tests.
Augmentin MFF and other medicines
Tell the doctor or pharmacist about all medicines that the child is currently taking or has recently taken, as well as any medicines that the child is to take.
If the child is also taking allopurinol (used for gout), it is more likely that they will experience skin allergic reactions.
If the child is taking probenecid (used for gout), the doctor may decide to modify the dose of Augmentin MFF.
If medicines that reduce blood clotting (such as warfarin) are given at the same time as Augmentin MFF, additional blood tests may be necessary.
Augmentin MFF may affect the action of methotrexate (a medicine used to treat cancer or rheumatic diseases).
Augmentin MFF may affect the action of mycophenolate mofetil (a medicine used to prevent transplant rejection).
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before taking this medicine.
Driving and using machines
Augmentin MFF may cause side effects and symptoms that can disrupt the ability to drive vehicles.
The patient should not drive vehicles or operate machines unless they feel well.
Augmentin MFF contains aspartame (E 951), benzyl alcohol, and maltodextrin
- This medicine contains 2.5 mg of aspartame (E 951) per 1 ml. Aspartame (E 951) is a source of phenylalanine. It may be harmful to patients with phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates in the body due to its improper excretion.
- Augmentin MFF contains trace amounts of benzyl alcohol. Benzyl alcohol may cause allergic reactions.
- Augmentin MFF contains maltodextrin (glucose). If the child being treated has previously been found to have intolerance to some sugars, they should consult a doctor before taking the medicine.
3. How to take Augmentin MFF
This medicine should always be taken as directed by the doctor or pharmacist. If in doubt, consult the doctor or pharmacist.
Adults and children weighing 40 kg or more
- This suspension is not usually recommended for use in adults and children weighing 40 kg or more. Consult a doctor or pharmacist for advice.
Children weighing less than 40 kg
All doses are determined based on the child's weight in kilograms.
- The doctor will recommend the dose of Augmentin MFF to be given to the child.
- The packaging may be provided with a plastic measuring spoon or a dosing syringe. These should be used to give the child the correct dose. The instructions for using the dosing syringe are at the end of this leaflet.
- Recommended dose - (25 mg + 3.6 mg)/kg body weight/day (0.4 ml of suspension/kg body weight/day) to (45 mg + 6.4 mg)/kg body weight/day (0.6 ml of suspension/kg body weight/day), given in two divided doses.
- Higher dose - up to (70 mg + 10 mg)/kg body weight/day (1.0 ml of suspension/kg body weight/day), given in two divided doses.
Patients with kidney and liver diseases
- If the child being treated has kidney disease, the dose of the medicine may be reduced. The doctor may choose a different dose of Augmentin MFF or a different medicine.
- If the child being treated has liver disease, they may have blood tests more frequently to check how the liver is functioning.
How to give Augmentin MFF
- Before giving each dose, the bottle should always be shaken well.
- Give at the start of a meal or just before a meal.
- Give at equal intervals of at least 4 hours between doses given during the day. Do not give 2 doses within 1 hour.
- Do not give Augmentin MFF to the child for more than 2 weeks. If the child still does not feel well, consult the doctor again.
Taking more than the recommended dose of Augmentin MFF
If a higher dose of Augmentin MFF than recommended is given to the child, symptoms such as stomach and intestinal irritation (nausea, vomiting, or diarrhea) or seizures may occur. Consult the doctor as soon as possible. Bring the medicine bottle with you to show the doctor.
Missing a dose of Augmentin MFF
If a dose of Augmentin MFF is missed, give it as soon as remembered. Do not give the next dose too early, but wait about 4 hours before giving the next dose.
Do not take a double dose to make up for a missed dose.
Stopping treatment with Augmentin MFF
Continue giving Augmentin MFF until the end of the treatment, even if the child feels better. All doses of the medicine are needed to fight the infection. If some bacteria survive, they may cause the infection to recur.
If you have any further doubts about taking this medicine, consult a doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following are side effects of this medicine that may occur:
Conditions to watch out for
Allergic reactions:
- skin rash
- vasculitis, which may appear as red or purple raised spots on the skin, but may also affect other organs
- fever, joint pain, swelling of the lymph nodes in the neck, armpit, or groin
- swelling, sometimes including the face or throat (angioedema), causing difficulty breathing
- fainting.
- If the child being treated experiences any of these symptoms, consult a doctor immediately. Stop taking Augmentin MFF.
Colitis
Colitis, causing watery diarrhea, usually with blood and mucus, abdominal pain, and (or) fever.
Drug-induced enterocolitis syndrome (DIES)
Drug-induced enterocolitis syndrome occurred mainly in children taking amoxicillin with clavulanic acid. It is a type of allergic reaction, the leading symptom of which is repeated vomiting (1 to 4 hours after taking the medicine). Further symptoms may include abdominal pain, lethargy, diarrhea, and low blood pressure.
Acute pancreatitis
If you have severe and persistent stomach pain, it may be a symptom of acute pancreatitis.
- If the child being treated experiences any of these symptoms, consult a doctor as soon as possible for advice.
Common side effects
May occur in up to 1 in 10 patients:
- thrush (candidiasis - fungal infections of the vagina, mouth, or skin folds)
- nausea, especially when taking high doses
- vomiting
- diarrhea (in children).
Uncommon side effects
May occur in up to 1 in 100 patients:
- skin rash, itching
- raised, itchy rash (hives)
- indigestion
- dizziness
- headache.
Uncommon side effects that may appear in blood test results:
- increased activity of certain substances (enzymes) produced in the liver.
Rare side effects
May occur in up to 1 in 1000 patients:
- skin rash, which may be accompanied by blisters and look like small targets (a dark spot in the center surrounded by a lighter ring with a dark ring around the edge - erythema multiforme)
Rare side effects that may appear in blood test results:
- low number of blood cells involved in blood clotting
- low number of white blood cells.
Side effects with unknown frequency
The frequency of occurrence cannot be determined from the available data.
- Allergic reactions (see above).
- Colitis (see above).
- Meningitis (aseptic meningitis).
- Severe skin reactions:
- widespread skin rash, which may be accompanied by blisters and peeling of the skin, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome), and a more severe form causing widespread peeling of the skin (more than 30% of the body surface - toxic epidermal necrolysis)
- widespread red skin rash, which may be accompanied by small pus-filled blisters (bullous exfoliative dermatitis)
- red scaly rash with thickening under the skin and blisters (acute generalized exanthematous pustulosis)
- flu-like symptoms with a rash, fever, swollen lymph nodes, and abnormal blood test results (including increased white blood cell count (eosinophilia) and increased liver enzyme activity); drug reaction with eosinophilia and systemic symptoms (DRESS).
- If the child being treated experiences any of these symptoms, consult a doctor immediately.
- Blistering rash arranged in a ring or like a string of pearls (linear IgA dermatosis).
- Hepatitis.
- Jaundice, caused by increased bilirubin levels in the blood, which can cause yellowing of the skin and eyes.
- Interstitial nephritis.
- Prolonged blood clotting time.
- Hyperactivity.
- Seizures (in people taking high doses of Augmentin MFF or with kidney disease).
- Black hairy tongue.
- Tooth discoloration (in children), which can usually be removed by brushing.
- Side effects that may appear in blood or urine test results:
- significantly reduced number of white blood cells
- low number of red blood cells (hemolytic anemia)
- crystals in the urine leading to acute kidney damage.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, you should tell the doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Reporting side effects can help gather more information on the safety of the medicine.
5. How to store Augmentin MFF
Keep the medicine out of the sight and reach of children.
Dry powder
Store in the original packaging to protect from moisture. Do not store above 25°C.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
Prepared suspension
The prepared suspension (medicine after adding water) should be stored in the refrigerator (at a temperature between 2°C and 8°C). Do not freeze.
The prepared suspension should be used within 7 days of preparation.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Augmentin MFF contains
- The active substances are amoxicillin and clavulanic acid. Each 1 ml of the prepared oral suspension contains 80 mg of amoxicillin as amoxicillin trihydrate and 11.4 mg of clavulanic acid as potassium clavulanate.
- Other ingredients are: aspartame (E 951), xanthan gum, anhydrous colloidal silica, hydrated colloidal silica, succinic acid, hypromellose, orange flavor 1, dry (containing maltodextrin and benzyl alcohol), orange flavor 2, dry (containing maltodextrin), raspberry flavor (containing maltodextrin), Golden Syrup flavor, dry (containing maltodextrin).
- Further information on aspartame (E 951), benzyl alcohol, and maltodextrin - see section 2.
What Augmentin MFF looks like and what the packaging contains
Augmentin MFF, (400 mg + 57 mg)/5 ml, powder for oral suspension is a white to off-white powder available in a colorless glass bottle. After adding water, the bottle contains 70 ml of a white suspension.
For more detailed information, consult the marketing authorization holder or parallel importer.
Marketing authorization holder in Portugal, the country of export:
GlaxoSmithKline – Produtos Farmacêuticos, Lda.
Rua Dr. António Loureiro Borges, 3
Arquiparque – Miraflores
1495-131 Algés
Portugal
Manufacturer:
Glaxo Wellcome Production
ZI de la Peyennière
53100 Mayenne
France
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warszawa
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warszawa
Marketing authorization number in Portugal, the country of export:5738422
Parallel import authorization number: 266/23
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland)
under the following names:
Austria – Augmentin 400 mg/57 mg/5 ml Pulver zur Herstellung einer Suspension zum Einnehmen
Multifruchtgeschmack
Bulgaria – Аугментин 400 mg/57 mg/5 ml прах за перорална суспензия
Croatia – Augmentin 400 mg + 57 mg/5 ml prašak za oralnu suspenziju s okusom voća
Cyprus – Augmentin Mixed Fruit
Czech Republic – Augmentin
Estonia – Augmentin Fruit
France – Augmentin 80 mg - 11.4 mg par ml Poudre pour suspension buvable
Greece – Augmentin MF
Germany – Augmentan Kindersaft
Hungary – Augmentin Duo
Ireland – Augmentin Duo Mixed Fruit
Italy – Augmentin
Latvia – Augmentin Fruit 400 mg/57 mg/5 ml pulveris iekšķīgi lietojamas suspensijas pagatavošanai
Lithuania – Augmentin
Malta – Augmentin Duo Mixed Fruit
Norway – Augmentin
Poland – Augmentin MFF
Portugal – Augmentin Duo
Romania – Augmentin FP
Slovakia – Augmentin DUO s príchuťou miešaného ovocia
United Kingdom (Northern Ireland) – Augmentin Duo.
Date of leaflet approval: 24.11.2023
[Information about the trademark]
Medical education
Antibiotics are used to treat bacterial infections. They are ineffective in treating viral infections.
Sometimes bacterial infections do not respond to antibiotic treatment.
One of the most common reasons for this phenomenon is that the bacteria are resistant to the antibiotic given.
This means that the bacteria can survive or multiply despite the use of the antibiotic.
Bacteria can become resistant to antibiotics for many reasons. Careful use of antibiotics can help reduce the possibility of bacteria developing resistance.
The antibiotic prescribed by the doctor is intended solely for the treatment of the current illness. Paying attention to the following tips will help prevent the development of resistant bacteria that could interfere with the action of the antibiotic.
- 1. It is very important to take the antibiotic in the correct dose, at the right time, and for the right number of days. Read the instructions in the patient leaflet and if any of them are unclear, ask the doctor or pharmacist to explain.
- 2. The patient should not take an antibiotic that was not prescribed for them. They should only take it to treat the infection for which the antibiotic was prescribed.
- 3. The patient should not take an antibiotic prescribed for another person, even if they had a similar infection.
- 4. Do not give antibiotics prescribed for one patient to another person.
- 5. If there are any remaining antibiotics after completing the treatment as recommended by the doctor, return them to the pharmacy, which will ensure their proper disposal.
Instructions for preparing the medicine for use
Open the bottle. Before use, check if the protective foil on the bottle has been damaged. Close the bottle.
- 1. Shake the medicine bottle to loosen the powder.
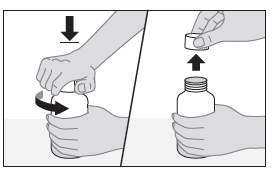
- 2. Open the bottle.
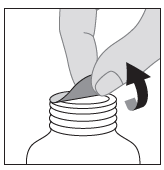
- 3. Tear off the protective foil.
- 4. Add water (the volume is indicated below). Close the bottle, turn it upside down, and shake well.
Alternative method of preparing the suspension: fill the bottle with water to a level just below the mark on the label. Close the bottle, turn it upside down, and shake well, then fill with water exactly to the mark. Close the bottle, turn it upside down, and shake well again.
- 5. The prepared suspension (medicine after adding water) should be stored in the refrigerator. Shake the bottle before each use. The prepared suspension should be used within 7 days.
Instructions for using the dosing syringe
The packaging may be provided with a dosing syringe. The dosing syringe is intended for oral administration of Augmentin MFF.
The dosing syringe should only be used to administer Augmentin MFF. It should not be used to administer other medicines, as the graduated scale is specific to Augmentin MFF.
The dosing syringe is provided with a connector that allows it to be connected to the bottle.
The dosing syringe for oral administration has a graduated scale that allows the required dose to be measured in milliliters (ml). Give the child the dose prescribed by the doctor.
Before use, check if the dosing syringe or connector is contaminated. If necessary, rinse them with clean water.
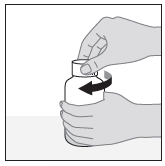
- 1. Shake the suspension bottle well before each use.
- 2. Remove the connector from the dosing syringe. Holding the bottle firmly, push the connector into the neck of the bottle (leave the connector in the neck of the bottle, do not remove it).
| Package size [ml] | Volume of water to add to prepare the suspension [ml] |
| 70 | 62 |
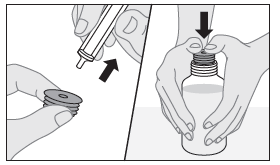
- 3. Insert the tip of the dosing syringe into the recess of the connector and make sure it is securely attached.
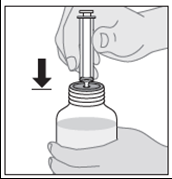
- 4. Turn the bottle upside down while holding the dosing syringe. Pull the plunger down to the dose of medicine prescribed by the doctor.
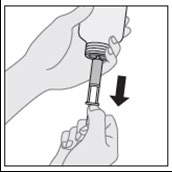
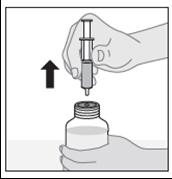
- 5. Turn the bottle right side up (neck up) and remove the dosing syringe from the connector.
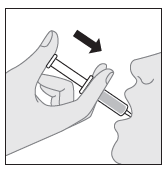
- 6. To give the medicine to the child, carefully place the tip of the dosing syringe in the child's mouth, then slowly press the plunger to give the suspension. (If it is necessary to give the prescribed dose in more than one dosing syringe, repeat the steps described in points 3, 4, 5, and 6).
- 7. After use, rinse the dosing syringe thoroughly with clean water. Leave it to dry completely before the next use.
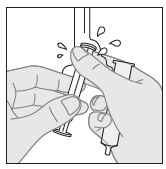
- 8. Close the bottle.
- 9. The prepared suspension (medicine after adding water) should be stored in the refrigerator. Shake the bottle before each use. The prepared suspension should be used within 7 days.
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)GlaxoSmithKline – Produtos Farmacêuticos, Lda.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Augmentin MffDosage form: Tablets, 875 mg + 125 mgActive substance: amoxicillin and beta-lactamase inhibitorPrescription not requiredDosage form: Powder, (600 mg + 42.9 mg)/5 mlActive substance: amoxicillin and beta-lactamase inhibitorManufacturer: Sandoz GmbHPrescription requiredDosage form: Powder, (600 mg + 42.9 mg)/5 mlActive substance: amoxicillin and beta-lactamase inhibitorPrescription required
Alternatives to Augmentin Mff in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Augmentin Mff in Spain
Alternative to Augmentin Mff in Ukraine
Online doctors for Augmentin Mff
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Augmentin Mff – subject to medical assessment and local rules.















