Artiss
Ask a doctor about a prescription for Artiss

How to use Artiss
Leaflet attached to the packaging: information for the user
ARTISS
Solutions for preparing tissue glue
Deep-frozen
Human fibrinogen, human thrombin, aprotinin, calcium chloride dihydrate
You should carefully read the contents of the leaflet before using the medicine, as it contains
important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- If the patient experiences any adverse reactions, including any adverse reactions not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is ARTISS and what is it used for
- 2. Important information before using ARTISS
- 3. How to use ARTISS
- 4. Possible side effects
- 5. How to store ARTISS
- 6. Package contents and other information
1. What is ARTISS and what is it used for
What is ARTISS
ARTISS is a two-component fibrin sealant and contains two of the proteins that make up a blood clot. These proteins are called fibrinogen and thrombin. When these proteins are mixed during administration, they form a clot at the site where the surgeon applies them.
ARTISS is prepared as two solutions (a protein glue solution and a thrombin solution) that are mixed during administration.
What is ARTISS used for
ARTISS is a tissue glue.
ARTISS is used to glue soft tissues in plastic, reconstructive, and burn surgery. For example, ARTISS can be used to glue skin grafts or skin flaps to burn wounds or in plastic surgery to glue skin to the underlying tissue. ARTISS can also be used to attach artificial skin to a wound site.
The clot formed by ARTISS is very similar to a natural blood clot. This means that it undergoes natural dissolution and does not leave any residue. Nevertheless, aprotinin (a protein that delays clot dissolution) is added to increase the durability of the clot and prevent its premature dissolution.
2. Important information before using ARTISS
Do not use ARTISS:
- If the patient is allergic to the active substances or any of the other ingredients of this medicine (listed in section 6).
- ARTISS should not be used to treat massive and severe bleeding.
- ARTISS should not be used as a substitute for skin sutures to close a surgical wound.
- ARTISS MUST NOT be injected into blood vessels (veins or arteries), or into tissues. Because ARTISS forms clots at the site of administration, injecting ARTISS can cause serious reactions (e.g., vessel closure). ARTISS should only be applied to tissue surfaces in a thin layer, where necessary.
- The patient should not be given ARTISS if they are allergic (hypersensitive) to the active substances, to bovine protein, or to any other component (see section 6) of ARTISS. This can cause serious allergic reactions. The patient should inform the surgeon or another doctor if they know they are allergic to aprotinin or any bovine protein.
- ARTISS spray application should not be used in endoscopic procedures. Use in laparoscopy (surgical procedures through body cavity openings), see section "Warnings and precautions".
Warnings and precautions
- Before starting to use ARTISS, the patient should discuss it with their doctor, pharmacist, or nurse.
- When using spray devices equipped with a pressure regulator to administer fibrin glues, life-threatening or fatal air or gas embolisms have been reported. It appears to be related to the use of the spray device at higher than recommended pressures and (or) closer than recommended to the tissue surface. The risk appears to be greater when fibrin glues are sprayed with air than with CO. Therefore, it cannot be excluded that such an event may occur due to the spraying of ARTISS.
- When applying ARTISS using a spray device, the pressure and spray distance must be within the range recommended by the device manufacturer. ARTISS should be administered strictly according to the instructions and only with devices recommended for use with this product.
- When spraying ARTISS, the patient should be monitored for changes in arterial pressure, heart rate, arterial oxygen saturation, and end-tidal CO concentration due to the possibility of air or gas embolism (see section 2).
- ARTISS should not be used with the EasySpray/Spray Set system in closed body areas for serious safety reasons.
- ARTISS is not recommended for laparoscopic procedures (surgical procedures performed through body cavity openings).
- ARTISS should only be applied using products marked with the CE mark.
- When using auxiliary tips with this product, the instructions for using these tips should be followed.
- If the patient has ever received ARTISS or aprotinin before, their body may have become sensitive to them. There is a possibility that the patient may be allergic to this product, even if no reactions occurred during the first administration. If the patient thinks they may have received one of these products during a previous operation, they should inform their doctor.
- If any symptoms of an allergic reaction occur, the doctor will immediately stop administering ARTISS and provide appropriate treatment.
- ARTISS is not indicated for supporting hemostasis and tissue gluing in cases where rapid clotting of the glue is required. ARTISS should not be used, in particular, in cardiac surgery where the glue is intended for vascular anastomoses.
- ARTISS is not indicated for use in neurosurgery or as a sealing material for intestinal or vascular anastomoses, as there is a lack of data to support these indications.
- Before applying ARTISS, the body areas outside the target application site should be sufficiently protected/covered to avoid unwanted tissue adhesion.
- ARTISS is applied in a thin layer. Excessive clot thickness may adversely affect the effectiveness of the product and the wound healing process.
- The doctor will not use oxycellulose-containing preparations as carrier materials, as they may reduce the effectiveness of ARTISS.
When medicines are manufactured from human blood or plasma, certain measures are taken to prevent the transmission of infections to patients. These measures include:
- careful selection of blood and plasma donors to ensure that those at risk of carrying infections are excluded,
- testing of individual blood samples and plasma pools for viruses/infections,
- incorporation of steps that inactivate or remove viruses during the processing of blood or plasma.
Despite these measures, when medicines are administered that are made from human blood or plasma, it cannot be completely excluded that the risk of transmission of infection cannot be completely ruled out. This also applies to unknown or newly discovered viruses and other types of infections.
The measures taken are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, as well as non-enveloped hepatitis A virus.
The measures taken may have limited value against non-enveloped viruses, such as parvovirus B19. Parvovirus B19 infection can be serious for pregnant women (fetal infection) and for individuals with impaired immune systems or with certain types of anemia (e.g., congenital spherocytosis or hemolytic anemia).
It is strongly recommended that each time a dose of ARTISS is administered to a patient, the name and batch number of the product should be recorded to keep track of the batches used.
ARTISS and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
ARTISS can be used at the same time as other medicines the patient is taking. There are no known interactions between ARTISS and other medicinal products.
Like other comparable preparations or thrombin solutions, the product may be destroyed by solutions containing alcohol, iodine, or heavy metals (e.g., disinfectant solutions).
Before applying the product, these types of substances should be thoroughly removed.
ARTISS with food and drink
The patient should ask their doctor. The doctor will decide whether the patient can eat and drink before administering ARTISS.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine. The doctor will decide whether ARTISS can be used during pregnancy or breastfeeding.
Driving and using machines
ARTISS has no effect on the ability to drive vehicles or operate machines.
ARTISS contains Polysorbate 80
Polysorbate 80 may cause skin allergy (e.g., rash, itching).
3. How to use ARTISS
- ARTISS is used only during surgical operations. The product can only be used by experienced surgeons who have been trained in this regard.
- The amount of ARTISS used depends on various factors, including the type of procedure, the size of the tissue surface to be treated, and the method of applying ARTISS. The surgeon will decide what amount is appropriate.
- During the operation, the surgeon will apply ARTISS to the appropriate tissue surface using a special device provided for application. This device ensures that equal amounts of both components of the fibrin glue are applied at the same time, which is important for achieving the optimal effect of ARTISS.
- Before applying ARTISS, the wound surface should be dried using standard techniques (e.g., changing compresses, gauzes, using suction devices).
- ARTISS can only be sprayed on visible surfaces.
- It is recommended that the initial application covers the entire area intended for treatment.
When applying ARTISS using a spray device, the following pressure and distance from the tissue should be observed:
| Recommended pressure and distance from the tissue values and spray device for applying ARTISS | |||||
| Spray set to be used | Applicator tips to be used | Pressure regulator to be used | Recommended distance from target tissue | Recommended spray pressure | |
| Treatment of open surgical wounds involving subcutaneous tissue | Spray set Tisseel/Artiss | nd. | EasySpray | 10–15 cm | 1.5–2.0 bar (21.5–28.5 psi) |
| Spray set Tisseel/Artiss — 10-piece package | nd. | EasySpray | |||
When spraying ARTISS, the patient should be monitored for changes in arterial pressure, heart rate, arterial oxygen saturation, and end-tidal CO concentration due to the possibility of air or gas embolism (see section 2).
Using a higher dose of ARTISS than recommended
ARTISS is used only during surgical operations. It is administered by the surgeon, and the amount of ARTISS used is also determined by the surgeon.
If the patient has any questions about using the medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, ARTISS can cause side effects, although not everybody gets them.
The following explains how the frequencies are defined in the following section:
Very common: may affect more than 1 in 10 people
Common: may affect up to 1 in 10 people
Uncommon: may affect up to 1 in 100 people
Rare: may affect up to 1 in 1,000 people
Very rare: may affect up to 1 in 10,000 people
Not known: frequency cannot be estimated from the available data
- There is a small possibility that the patient may experience an allergic reaction to one of the components of ARTISS (see section 6). This is more likely if the patient has been treated with ARTISS or aprotinin during a previous operation. Allergic reactions can be severe, and it is very important to discuss the possibility of their occurrence with the doctor.
- Anaphylactic/anaphylactoid reactions may occur, with an unknown frequency. Early symptoms of allergic reactions may include: sudden redness, low blood pressure, rapid or slow heart rate, nausea (feeling sick), hives, itching, difficulty breathing.
- The surgical team is aware of the risk of this type of reaction - if they notice any symptoms, they will immediately stop administering ARTISS. Severe symptoms may require emergency measures. The frequency of allergic reactions is unknown.
- Injecting ARTISS into soft tissues may cause local tissue damage. The frequency is unknown.
- Injecting ARTISS into blood vessels (veins or arteries) may cause thrombosis (thrombosis). The frequency is unknown.
- Since ARTISS is made from plasma from human blood donors, it cannot be completely excluded that the risk of transmission of infection cannot be completely ruled out, but the manufacturer takes numerous measures to reduce this risk (see section 2).
- Life-threatening or fatal air or gas embolism cases have occurred with the use of spray devices using pressure regulators to administer fibrin glues. It appears to be related to the use of the spray device at higher than recommended pressures and (or) closer than recommended to the tissue surface.
Adverse reactions reported during clinical trials of ARTISS and after the product was placed on the market are listed below. The frequencies of adverse reactions are based on data from a controlled clinical trial involving 138 patients, in which ARTISS was used to attach skin grafts to burn wounds. None of the adverse reactions observed in the clinical trial were classified as severe.
| Table 1 Adverse reactions | |
| Adverse reaction | Frequency |
| Skin cyst | Uncommon |
| Itching | Common |
| Skin graft failure | Common |
| Air or gas bubbles in the vascular system (air embolism)* | Not known |
*the occurrence of air or gas bubbles in the vascular system occurred when fibrin glues were applied using compressed air or gas; it appears to be related to the improper use of the spray device (e.g., at higher than recommended pressure and (or) closer than recommended to the tissue surface).
The following adverse reactions have been reported for other tissue glues, but their frequency cannot be determined: allergy, severe allergic reaction, slow heart rate, rapid heart rate, low blood pressure, bleeding, shallow breathing, nausea, hives, sudden redness, wound healing disorder, swelling, fever, and fluid accumulation under the skin at the treatment site.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the patient should tell their doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects can help gather more information on the safety of the medicine.
5. How to store ARTISS
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date stated on the label after "EXP".
- Store and transport at a frozen state (at a temperature of ≤ -20°C), in unchanged conditions, until ready to use.
- Store the syringe in its original packaging to protect it from light.
Storage after thawing
The product in unopened protective bags, thawed at room temperature, can be stored for up to 14 days at a controlled room temperature (not exceeding 25°C).
After thawing, do not refreeze or refrigerate!
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What ARTISS contains
ARTISS contains two components:
Component 1 = Protein glue solution:
The active substances in 1 ml of protein glue solution are:
Human fibrinogen, 91 mg/ml, produced from human plasma; synthetic aprotinin 3000 KIU/ml
The excipients are: human albumin, L-histidine, nicotinamide, polysorbate 80, sodium citrate dihydrate, and water for injections.
Component 2 = Thrombin solution:
The active substances in 1 ml of thrombin solution are:
Human thrombin, 4 IU/ml, produced from human plasma; calcium chloride dihydrate, 40 µmol/ml
The excipients are: human albumin, sodium chloride, and water for injections.
| After mixing | 1 ml | 2 ml | 4 ml | 10 ml |
| Component 1: Protein glue solution Human fibrinogen (as clotting protein) Aprotinin (synthetic) | 45.5 mg 1500 KIU | 91 mg 3000 KIU | 182 mg 6000 KIU | 455 mg 15000 KIU |
| Component 2: Thrombin solution Human thrombin Calcium chloride dihydrate | 2 IU 20 µmol | 4 IU 40 µmol | 8 IU 80 µmol | 20 IU 200 µmol |
ARTISS contains human factor XIII, co-purified with human fibrinogen, in amounts of 0.6–5 IU/ml.
What ARTISS looks like and contents of the pack
Solutions for preparing tissue glue.
Frozen solutions for preparing tissue glue (1 ml, 2 ml, or 5 ml protein glue solution and 1 ml, 2 ml, or 5 ml thrombin solution in a dual-chamber syringe for single use, packaged in a bag).
Pack size: 1 piece.
Contents of the packaging with PRIMA syringe:
1 ml, 2 ml, or 5 ml protein glue solution and 1 ml, 2 ml, or 5 ml thrombin solution in a filled dual-chamber (polypropylene) syringe for single use, closed with a stopper and packaged in two bags with a set consisting of 2 connectors and 4 application needles.
Contents of the packaging with AST syringe:
1 ml, 2 ml, or 5 ml protein glue solution and 1 ml, 2 ml, or 5 ml thrombin solution in a filled dual-chamber (polypropylene) syringe for single use, closed with a stopper and packaged in two bags with a set consisting of 1 double syringe plunger, 2 connectors, 4 application needles.
The solution is colorless or pale yellow.
Not all pack sizes may be marketed.
Marketing authorization holder
Baxter Polska Sp. z o.o.
ul. Kruczkowskiego 8
00-380 Warsaw
Manufacturer
Takeda Manufacturing Austria AG
Industriestrasse 67
A-1221 Vienna
Austria
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
ARTISSin the following countries: Austria, Belgium, Czech Republic, Germany, Greece, Spain, Finland, France, Ireland, Italy, Luxembourg, Netherlands, Norway, Poland, Portugal, United Kingdom (Northern Ireland)
Artiss:Denmark, Iceland, Sweden
Date of last revision of the leaflet:January 2022
------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Fertility, pregnancy, and lactation
No controlled clinical trials have been conducted to determine the safety of using fibrin glues/hemostatic agents in pregnant or breastfeeding women. No studies have been conducted in animals.
For this reason, the product should only be used in pregnant or breastfeeding women if it is clearly necessary.
The effect of ARTISS on fertility has not been established.
Dosage and administration
ARTISS can only be used in a hospital setting. The product can only be used by experienced surgeons who have been trained in this regard.
Dosage
The volume and frequency of application of ARTISS should always be adapted to the individual needs of the patient, depending on the clinical situation.
The dose to be used depends on various variables, including the type of surgical procedure, the size of the tissue surface to be treated, and the chosen method of applying the glue and the number of applications.
The application of the product must be determined individually by the treating physician. In clinical trials, individually determined doses were usually between 0.2 and 12 ml. In some procedures (e.g., treating extensive burn wounds), it may be necessary to use larger volumes of the product.
The initial dose of the product applied to the selected anatomical site or target surface should be sufficient to completely cover the intended application area. If necessary, the application of the product can be repeated on small areas that may not have been covered previously. However, it should be avoided to reapply ARTISS to previously existing polymerized ARTISS, as ARTISS will not adhere to the polymerized layer.
It is recommended that the initial application covers the entire area intended for treatment.
| Approximate surface to be glued tissue | Required package size of ARTISS |
| 100 cm2 200 cm2 500 cm2 | 2 ml 4 ml 10 ml |
To avoid excessive granulation and to ensure gradual absorption of the solidified glue, only very thin layers of the protein glue-thrombin mixture should be applied.
ARTISS has not been used in clinical trials in people over 65 years old.
Pediatric population
Currently available data are described in section 5.1 of the Summary of Product Characteristics, but no dosage recommendations can be provided.
Administration
Locally (topically). Do not inject.
Only for subcutaneous administration. ARTISS is not recommended for use in laparoscopic procedures.
To ensure optimal safety conditions when using ARTISS, it should be applied using a device equipped with a pressure regulator that generates a maximum pressure of 2.0 bar (28.5 psi).
Before applying ARTISS, the wound surface should be dried using standard techniques (e.g., changing compresses, gauzes, using suction devices). Compressed air or gas should not be used to dry the surface.
ARTISS can only be sprayed on visible surfaces.
The product should be dissolved and administered according to the instructions and using devices recommended for use with this product.
When spraying the product, see below under Administration.
Special precautions for disposal and preparation of the medicinal product for use (packaging: PRIMA syringe)
General information
- Before applying ARTISS, the body areas outside the target application site should be sufficiently protected/covered to avoid unwanted tissue adhesion.
- To prevent ARTISS from sticking to gloves and surgical instruments, they should be moistened with a saline solution before coming into contact with the glue.
- As a guideline for gluing surfaces, it can be assumed that 1 set of ARTISS glue 2 ml (i.e., 1 ml protein glue solution plus 1 ml thrombin solution) is sufficient to cover an area of at least 10 cm².
- The required dose depends on the size of the surface to be glued.
- DO NOT use the two components of ARTISS separately. Both components must be used together.
- DO NOT expose ARTISS to temperatures above 37°C. DO NOT heat in a microwave.
- DO NOT thaw ARTISS by holding it in your hands.
- DO NOT use ARTISS until it has been completely thawed and warmed to a temperature of 33°C - 37°C.
- Remove the protective cap from the syringe only after thawing and warming. To facilitate removal of the protective cap from the syringe, move the protective cap back and forth and then remove the protective cap from the syringe.
- Remove all air from the syringe and then attach the connector and application needle.
Preparation for use
The inner bag and its contents are sterile, unless the integrity of the outer part of the packaging has been compromised. Using aseptic technique, transfer the sterile inner bag and its contents to a sterile area.
The ready-to-use syringe can be thawed AND warmed using one of the following methods:
- 1. Rapid thawing/warming (sterile water bath) – recommended method
- 2. Thawing/warming in a non-sterile water bath
- 3. Thawing/warming in an incubator
- 4. The ready-to-use syringe can also be thawed and stored at room temperature (not exceeding 25°C) for up to 14 days. Before use, it must be warmed.
1. Rapid thawing/warming (sterile water bath) – recommended method:
It is recommended to thaw and warm both components of the glue using a sterile water bath at a temperature of 33°C - 37°C.
- The water bath temperature should not exceed 37°C. To control the specified temperature range, it is necessary to control the water temperature using a thermometer and change the water if necessary.
- If a sterile water bath is used for thawing and warming, the syringe should be removed from the bag before placing it in the sterile water bath.
Instructions:
Transfer the inner bag to sterile conditions, remove the ready-to-use syringe from the inner bag, and place it directly in the sterile water bath. Ensure that the contents of the ready-to-use syringe are completely submerged in water.
Table 1: Minimum thawing and warming time in a sterile water bath
| Package size | Minimum thawing/warming time 33°C to 37°C, sterile water bath product removed from bags |
| 2 ml | 5 minutes |
| 4 ml | 5 minutes |
| 10 ml | 10 minutes |
2. Thawing/warming in a non-sterile water bath
Instructions:
Place the ready-to-use syringe in both protective bags in a water bath outside the sterile area for the appropriate time (see Table 2). Ensure that the bags remain submerged in water during the entire thawing time. After thawing, remove the bags from the water bath, dry the outer bag, and transfer the inner bag with the ready-to-use syringe to the sterile area.
Table 2: Minimum thawing and warming time in a non-sterile water bath
| Package size | Minimum thawing/warming time 33°C to 37°C, non-sterile water bath product in bags |
| 2 ml | 15 minutes |
| 4 ml | 20 minutes |
| 10 ml | 35 minutes |
3. Thawing/warming in an incubator
Instructions:
Place the ready-to-use syringe in both protective bags in an incubator outside the sterile area for the appropriate time (see Table 3). After thawing/warming, remove the bags from the incubator, remove the outer bag, and transfer the inner bag with the ready-to-use syringe to the sterile area.
Table 3: Minimum thawing and warming time in an incubator
| Package size | Minimum thawing/warming time 33°C to 37°C, incubator product in bags |
| 2 ml | 40 minutes |
| 4 ml | 50 minutes |
| 10 ml | 90 minutes |
4. Thawing at room temperature (not exceeding 25°C) BEFORE warming
Instructions:
Thaw the ready-to-use syringe in both protective bags at room temperature outside the sterile area for the appropriate time (see Table 4). After thawing, to warm the product for use, warm it in the outer bag in an incubator. After thawing at room temperature, the maximum storage time for the product (in both bags) at room temperature is 14 days.
Table 4: Minimum thawing time at room temperature outside the sterile area and additional warming time in an incubator to a temperature of 33°C to 37°C
| Package size | Minimum thawing time of the product at room temperature (not exceeding 25°C) and warming time in an incubator before use, to a temperature of 33°C to 37°C Product in bags | |
| Thawing at room temperature (not exceeding 25°C) | Warming in incubator (33°C-37°C) | |
| 2 ml | 80 minutes | + 11 minutes |
| 4 ml | 90 minutes | + 13 minutes |
| 10 ml | 160 minutes | + 25 minutes |
Stability after thawing
After thawing and warming(at a temperature of 33°C to 37°C, methods 1, 2, and 3), the chemical and physical stability of the product has been demonstrated for 4 hours at a temperature of 33°C to 37°C.
For the product thawedat room temperature, in an unopened bag (method 4), the chemical and physical stability of the product has been demonstrated for 14 days at a temperature not exceeding 25°C.
Warm to a temperature of 33°C to 37°C immediately before use.
From a microbiological point of view, the product should be used immediately after warming to a temperature of 33°C to 37°C, unless the opening/thawing method excludes the risk of microbiological contamination.
If the product is not used immediately, the user is responsible for the storage time and conditions.
Do not refreeze or refrigerate after starting thawing.
Handling the product after thawing/before administration
To achieve optimal mixing of the two solutions and optimal clotting of the fibrin glue, the temperature of both glue components should be maintained at 33°C - 37°C until application.
The protein glue solution and thrombin solution should be clear or slightly opalescent.
Do not use cloudy solutions or those containing sediment. Thawed products should be visually inspected for the presence of insoluble particles and changes in color or any change in physical appearance before use. If any of the above occur, the solution should be discarded.
The thawed protein glue solution should be a slightly viscous liquid. If the solution has a solid gel-like consistency, it should be assumed that it has denatured (probably due to a break in the cold chain during storage or overheating during warming). In this case, ARTISS MUST NOT be used under any circumstances.
- Remove the syringe from the bags immediately before use.
- Use ARTISS only after it has been completely thawed and warmed (liquid consistency).
- Remove the protective cap from the syringe only immediately before application. PRIMA syringe: To facilitate removal of the protective cap from the syringe, move the protective cap back and forth and then remove the protective cap from the syringe.
Non-spray application using the PRIMA syringe:
To apply the glue, connect the dual-chamber ready-to-use syringe filled with protein glue solution and thrombin solution to the connector and application needle provided in the attached set of instruments. The common plunger of the dual-chamber ready-to-use syringe ensures that equal amounts of both glue components are administered to the connector, which are then mixed in the application needle and applied.
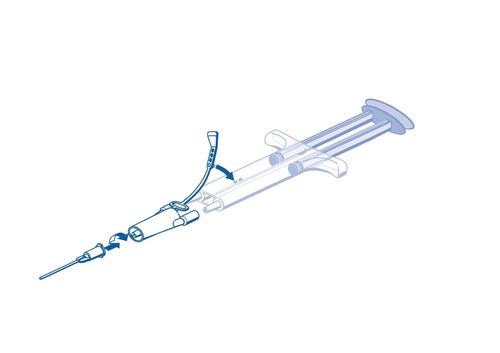
Safety strap
Double plunger
Dual-chamber syringe
Connecting element
Application needle
- Remove all air from the syringe before connecting it to the administration device
- Connect the connector and safety strap to the side of the syringe with the opening for the safety strap.
- Connect the outlets of the dual-chamber ready-to-use syringe to the connector, ensuring they are properly secured. o Secure the connector by attaching the safety strap to the ready-to-use dual-chamber syringe. o If the safety strap is broken, use the additional connector contained in the set. o If there is no additional connector, the set can still be used if it is ensured that the connection is stable and tight. o DO NOT remove air from the connector.
- Attach the application needle to the connector. o DO NOT remove air from the connector or application needle before starting the actual glue application, as this may cause the needle to become clogged.
Administration
Before applying ARTISS, the wound surface should be dried using standard techniques (e.g., changing compresses, gauzes, using suction devices). Compressed air or gas should not be used to dry the surface.
- Apply the mixed protein glue-thrombin solution to the target surface or surfaces to be glued, slowly pressing the rear part of the common plunger.
- In surgical procedures that require minimal amounts of fibrin glue, it is recommended to express and discard the first few drops of the product.
- After applying ARTISS, wait at least 3 minutes to achieve sufficient polymerization.
Caution: If the application of the glue components is interrupted, the needle may become clogged immediately. In this case, the application needle should be replaced with a new one immediately before reapplying the glue. If the connector outlets are clogged, the additional connector contained in the packaging should be used.
Application is also possible using other instruments provided by BAXTER, which are specially designed for applying to large or hard-to-reach surfaces. When using such application instruments, the instructions for using them should be followed carefully.
To obtain further instructions for preparation, the patient should consult their nurse or doctor.
Applying the Product Using a Spray Device
The pressure regulator should be used in accordance with the manufacturer's instructions.
When applying the ARTISS product using a spray device, the pressure and distance from the tissue should be observed in accordance with the manufacturer's recommendations, as follows:
| Recommended Pressure and Distance Values from Tissue and Spray Device for Applying the ARTISS Product | |||||
| Spray Set to be Used | Applicator Tips to be Used | Pressure Regulator to be Used | Recommended Distance from Target Tissue | Recommended Spray Pressure | |
| Surgical Treatment of Open Wound Subcutaneous Tissue | Tisseel/Artiss Spray Set | nd. | EasySpray | 10–15 cm | 1.5–2.0 bar (21.5–28.5 psi) |
| Tisseel/Artiss Spray Set — 10-piece packaging | nd. | EasySpray | |||
During Spraying of the ARTISS Product, Monitor Changes in Arterial Pressure, Pulse, Arterial Blood Oxygen Saturation, and End-Tidal CO2 Concentration Due to the Possibility of Air or Gas Embolism (See Summary of Product Characteristics
sections 4.2 and 4.4).
If auxiliary tips are used with this product, follow the instructions for using the tips.
Removal of Residues
Any unused residues of the medicinal product or its waste should be disposed of in accordance with local regulations.
Special Precautions for Disposal and Preparation of the Medicinal Product for Use (AST Syringe Packaging)
General Information
- Before applying ARTISS, protect the body areas outside the target application site to avoid unwanted tissue adhesion.
- To prevent ARTISS from sticking to gloves and surgical instruments, moisten them with physiological saline solution before coming into contact with the adhesive.
- As a guideline for gluing surfaces, assume that 1 set of ARTISS 2 ml adhesive (i.e., 1 ml of protein solution plus 1 ml of thrombin solution) is sufficient to cover an area of at least 10 cm2.
- The required dose depends on the size of the glued surface.
- DO NOT use the two components of the ARTISS product separately. Both components must be used together.
- DO NOT expose the ARTISS product to temperatures above 37°C. DO NOT subject to microwave radiation.
- DO NOT thaw the product by holding it in your hands.
- DO NOT use the ARTISS product until it has been completely thawed and warmed to a temperature of 33°C - 37°C.
- Remove the protective cap from the syringe only after thawing and warming have been completed.
- Remove all air from the syringe and then attach the connector and application needle.
Preparation for Use
The inner bag and its contents are sterile, unless the integrity of the outer packaging has been compromised. Using aseptic technique, transfer the sterile inner bag and its contents to a sterile area.
The ready-to-use syringe can be thawed AND warmed using one of the following methods:
- 1. Rapid Thawing/Warming (Sterile Water Bath) – Recommended Method
- 2. Thawing/Warming in a Non-Sterile Water Bath
- 3. Thawing/Warming in an Incubator
- 4. The ready-to-use syringe can also be thawed and stored at room temperature (not exceeding 25°C) for a maximum of 14 days. Before use, warming is required.
- 1) Rapid Thawing/Warming (Sterile Water Bath) – Recommended Method
It is recommended to thaw and warm both components of the adhesive using a sterile water bath at a temperature of 33°C - 37°C.
- The temperature of the water bath should not exceed 37°C. To control the specified temperature range, the water temperature should be continuously monitored using a thermometer, and the water changed as necessary.
- If a sterile water bath is used for thawing and warming, remove the syringe from the bag before placing it in the sterile water bath.
Instructions:
Transfer the inner bag to a sterile environment, remove the ready-to-use syringe from the inner bag, and place it directly in the sterile water bath. Ensure that the contents of the ready-to-use syringe are completely submerged in water.
Table 1: Minimum Thawing and Warming Time in a Sterile Water Bath
Minimum Thawing/Warming Time
Package Size
33°C to 37°C, Sterile Water Bath Product Removed from Bags
2 ml
5 minutes
4 ml
5 minutes
10 ml
12 minutes
- 2) Thawing/Warming in a Non-Sterile Water Bath
Instructions:
Place the ready-to-use syringe in both protective bags in a water bath outside the sterile area for the appropriate time (see Table 2). Ensure that the bags remain submerged in water during the entire thawing time. After thawing, remove the bags from the water bath, dry the outer bag, and transfer the inner bag with the ready-to-use syringe and plunger to a sterile area.
Table 2: Minimum Thawing and Warming Time in a Non-Sterile Water Bath
Minimum Thawing/Warming Time
Package Size
33°C to 37°C, Non-Sterile Water Bath Product in Bags
2 ml
30 minutes
4 ml
40 minutes
10 ml
80 minutes
- 3) Thawing/Warming in an Incubator
Instructions:
Place the ready-to-use syringe in both protective bags in an incubator outside the sterile area for the appropriate time (see Table 3). After thawing/warming, remove the bags from the incubator, remove the outer bag, and transfer the inner bag with the ready-to-use syringe to a sterile area.
Table 3: Minimum Thawing and Warming Time in an Incubator
Minimum Thawing/Warming Time
Package Size
33°C to 37°C, Incubator Product in Bags
2 ml
40 minutes
4 ml
85 minutes
10 ml
105 minutes
- 4) Thawing at Room Temperature (Not Exceeding 25°C) BEFORE Warming
Instructions:
Thaw the ready-to-use syringe in both protective bags at room temperature outside the sterile area for the appropriate time (see Table 4). After thawing, to warm the product for use, warm it in the outer bag in an incubator. After thawing at room temperature, the maximum storage time for the product (in both bags) at room temperature is 14 days.
Table 4: Minimum Thawing Time at Room Temperature Outside the Sterile Area and Additional Warming Time in an Incubator to a Temperature of 33°C to 37°C
Minimum Thawing Time of the Product at Room Temperature (Not Exceeding 25°C) and Warming Time in an Incubator, Before Use, to a Temperature of up to 37°C
Maximum
Product in Bags
Thawing at Room Temperature (Not Exceeding 25°C)
Package Size
Warming in an Incubator (33°C-37°C)
2 ml
80 minutes
+ 11 minutes
4 ml
110 minutes
+ 25 minutes
10 ml
160 minutes
+ 35 minutes
Stability After Thawing
After thawing and warming(at a temperature of 33°C to 37°C, methods 1, 2, and 3), the chemical and physical stability of the product has been demonstrated for 4 hours at a temperature of 33°C to 37°C.
For the product thawedat room temperature, in an unopened bag (method 4), the chemical and physical stability of the product has been demonstrated for 14 days at a temperature not exceeding 25°C.
Warm to a temperature of 33°C to 37°C immediately before use.
From a microbiological point of view, the product should be used immediately after warming to a temperature of 33°C to 37°C, unless the opening/thawing method excludes the risk of microbiological contamination.
If the product is not used immediately, the user is responsible for the time and conditions of storage.
Do not re-freeze or refrigerate after thawing has begun.
Handling the Product After Thawing/Before Administration
To achieve optimal mixing of the two solutions and optimal coagulation of the fibrin glue, keep the temperature of both adhesive components at 33°C - 37°C untilapplication.
The protein solution and thrombin solution should be clear or slightly opalescent. Do not use cloudy solutions or those containing sediment. Thawed products should be visually inspected for the presence of insoluble particles and changes in color or any change in physical appearance before use. If any of these conditions occur, the solution should be discarded.
The thawed protein solution should be a slightly viscous liquid. If the solution has a solid gel-like consistency, it should be assumed that it has denatured (probably due to a break in the cold chain or overheating during warming). In this case, DO NOT use ARTISS under any circumstances.
- Do not remove the syringe from the bag until the time of use.
- Use ARTISS only after complete thawing and warming (liquid consistency).
- Remove the protective cap from the syringe just before applying.
Non-Spray Application Using the AST Syringe:
To apply the adhesive, connect the dual-chamber ready-to-use syringe filled with the protein solution and thrombin solution to the connector and application needle provided in the attached instrument set. The common plunger of the dual-chamber ready-to-use syringe, provided in the set of application devices, ensures the delivery of equal volumes of both components, which are then mixed in the application needle and applied.
AST Syringe Operating Instructions:
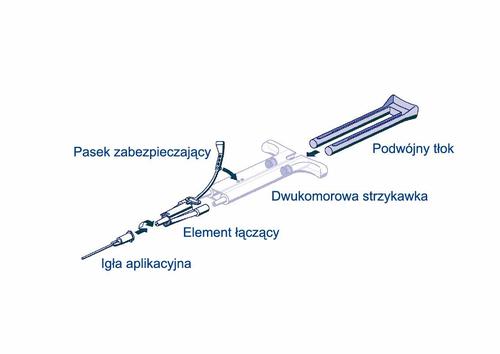
- Remove all air from the syringe before connecting to the delivery device.
- Connect the connector and strap to the side of the syringe with the strap safety opening.
- Connect the outlets of the dual-chamber ready-to-use syringe to the connector, ensuring they are properly secured. o Secure the connector by attaching the safety strap to the ready-to-use dual-chamber syringe. o If the safety strap is broken, use the additional connector provided in the packaging. o If there is no additional connector, the packaging can still be used if care is taken to ensure the connection is secure and leak-tight. o DO NOT remove air from the connector.
- Attach the application needle to the connector. o DO NOT remove air from the connector or application needle before starting the actual application, as this may cause the needle to become clogged.
Application
Before applying the ARTISS product, dry the wound surface using standard techniques (e.g., changing compresses, gauzes, using suction devices). Do not use compressed air or gas to dry the surface.
- Apply the mixed protein-thrombin solution to the target surface or surfaces to be glued, slowly pressing the rear part of the common plunger.
- In surgical procedures that require minimal amounts of fibrin glue, it is recommended to express and discard the first few drops of the product.
- After applying ARTISS, wait at least 3 minutes to achieve sufficient polymerization.
Note: If the application of the fibrin glue components is interrupted, the needle may become clogged immediately. In this case, the application needle should be replaced with a new one immediately before reapplying the glue. If the connector outlets are clogged, use the additional connector provided in the packaging.
Application is also possible using other instruments provided by BAXTER, which are specifically designed for applying to large or hard-to-reach surfaces. When using such instruments for application, follow their operating instructions carefully.
For further instructions on preparation, consult a nurse or doctor.
Applying the Product Using a Spray Device
The pressure regulator should be used in accordance with the manufacturer's instructions.
When applying the ARTISS product using a spray device, the pressure and distance from the tissue should be observed in accordance with the manufacturer's recommendations, as follows:
Recommended Pressure and Distance Values from Tissue and Spray Device for Applying the ARTISS Product
Spray Set to be Used
Końcówki Aplikatora, które mają być zastosowane
Regulator Ciśnienia, który ma być zastosowany
Zalecana Odległość od Tkanki Docelowej
Zalecane Ciśnienie Rozpylania
Tisseel/Artiss Spray Set
nd.
EasySpray
Leczenie Chirurgiczne Rany Otwartej Tkanki Podskórnej
1.5–2.0 bar (21.5–28.5 psi)
10–15 cm
Tisseel/Artiss Spray Set — Opakowanie 10 Szt.
During Spraying of the ARTISS Product, Monitor Changes in Arterial Pressure, Pulse, Arterial Blood Oxygen Saturation, and End-Tidal CO2 Concentration Due to the Possibility of Air or Gas Embolism (See Summary of Product Characteristics
sections 4.2 and 4.4).
If auxiliary tips are used with this product, follow the instructions for using the tips.
Removal of Residues
Any unused residues of the medicinal product or its waste should be disposed of in accordance with local regulations.
- Country of registration
- Prescription requiredNo
- ImporterTakeda Manufacturing Austria AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
Online doctors for Artiss
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Artiss – subject to medical assessment and local rules.