

Aprokam

Ask a doctor about a prescription for Aprokam

How to use Aprokam
Package Leaflet: Information for the Patient
APROKAM, 50 mg, Powder for Solution for Injection
Cefuroxime
Read the Package Leaflet Carefully Before Using the Medicinal Product, as it Contains Important Information for the Patient.
- Keep this Package Leaflet, You May Need to Read it Again.
- If You Have any Further Questions, Ask Your Doctor, Pharmacist, or Nurse.
- This Medicinal Product has been Prescribed for You. Do not Pass it on to Others. It may Harm them, even if their Symptoms are the Same as Yours.
- If You Experience any Side Effects, including those not Listed in this Package Leaflet, Tell Your Doctor, Pharmacist, or Nurse. See Section 4.
Package Leaflet Contents:
- 1. What is APROKAM and What is it Used for
- 2. Important Information Before Using APROKAM
- 3. How to Use APROKAM
- 4. Possible Side Effects
- 5. How to Store APROKAM
- 6. Package Contents and Other Information
1. What is APROKAM and What is it Used for
- APROKAM Contains the Active Substance Cefuroxime (as Cefuroxime Sodium), which Belongs to a Group of Antibiotics Called Cephalosporins. Antibiotics are Used to Kill Bacteria or "Pathogenic Microorganisms" that Cause Infections.
- This Medicinal Product will be Used when the Patient Undergoes Cataract Surgery (Clouding of the Lens).
- The Eye Surgeon will Administer this Medicinal Product by Injection into the Eye at the End of the Surgical Procedure (Cataract Surgery) to Prevent Eye Infection.
2. Important Information Before Using APROKAM
When not to Use APROKAM
- If the Patient is Allergic to Cefuroxime or any other Antibiotic of the Cephalosporin Group.
Warnings and Precautions
Before Using APROKAM, Tell Your Doctor, Pharmacist, or Nurse:
- If the Patient is Allergic to other Antibiotics, such as Penicillin,
- If the Patient is at Risk of Infection Caused by Bacteria Called Methicillin-Resistant Staphylococcus aureus,
- If the Patient is at Risk of Severe Infection,
- If the Patient has Complicated Cataract,
- If a Combined Eye Surgical Procedure is Planned,
- If the Patient has Severe Thyroid Disease.
APROKAM is Administered Exclusively by Injection into the Eye (Injection into the Anterior Chamber of the Eye).
APROKAM should be Administered in Aseptic Conditions (Clean and Free from Microorganisms Environment) during Cataract Surgery.
Each Vial of APROKAM is Intended for Use in a Single Patient Only.
APROKAM and Other Medicinal Products
Tell Your Doctor or Pharmacist about all Medicinal Products the Patient is Taking or has Recently Taken, as well as any Medicinal Products the Patient Plans to Use.
Pregnancy and Breastfeeding
- If the Patient is Pregnant or Breastfeeding, Thinks she may be Pregnant or is Planning to have a Child, she should Consult her Doctor or Pharmacist before Using this Medicinal Product.
- APROKAM will be Administered to the Patient only if the Benefits Outweigh the Potential Risks.
APROKAM Contains Sodium
The Medicinal Product Contains Less than 1 mmol (23 mg) of Sodium per Dose, which Means the Medicinal Product is Considered "Sodium-Free".
3. How to Use APROKAM
- APROKAM Injections will be Administered by the Eye Surgeon at the End of the Cataract Surgical Procedure.
- APROKAM is Supplied as a Sterile (Aseptic) Powder and is Dissolved in a Sodium Chloride Injection Solution before Administration.
Using More than the Recommended Dose or Too Little of APROKAM
The Medicinal Product will Usually be Administered by Medical Personnel.
If You have any Further Questions about the Use of this Medicinal Product, Ask Your Doctor, Pharmacist, or Nurse.
4. Possible Side Effects
Like all Medicinal Products, APROKAM can Cause Side Effects, although not Everybody gets them.
The Following Side Effect Occurs Very Rarely (may Affect up to 1 in 10,000 People):
- Severe Allergic Reaction, which Causes Difficulty Breathing or Dizziness.
The Following Side Effect is Reported with a Frequency of "Unknown" (Frequency cannot be Estimated from the Available Data):
- Macular Edema (Blurred or Wavy Vision in the Center of the Visual Field or its Vicinity).
Reporting Side Effects
If You Experience any Side Effects, including those not Listed in this Package Leaflet, Tell Your Doctor, Pharmacist, or Nurse. Side Effects can be Reported Directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw,
tel.: +48 22 49 21 301, fax: +48 22 49 21 309.
e-mail: [email protected]
Side Effects can also be Reported to the Marketing Authorization Holder.
By Reporting Side Effects, You can Help Provide more Information on the Safety of this Medicinal Product.
5. How to Store APROKAM
Store the Medicinal Product out of the Sight and Reach of Children.
Do not Use APROKAM after the Expiration Date Stated on the Carton and Vial Label after EXP. The Expiration Date refers to the Last Day of the Month.
Store in a Temperature below 25°C. Store the Vial in the Outer Packaging to Protect from Light.
For Single Use Only.
After Preparation of the Solution: the Product should be Used Immediately.
Medicinal Products should not be Disposed of via Wastewater or Household Waste. Ask Your Pharmacist how to Dispose of Medicinal Products no Longer Required. This will Help Protect the Environment.
6. Package Contents and Other Information
What APROKAM Contains
The Active Substance is Cefuroxime (as Cefuroxime Sodium).
One Vial Contains 50 mg of Cefuroxime.
After Preparation of the Solution, 0.1 ml of the Solution Contains 1 mg of Cefuroxime.
The Medicinal Product does not Contain any other Ingredients.
What APROKAM Looks Like and Contents of the Package
APROKAM is a White to Almost White Powder for Solution for Injection, Supplied in Glass Vials.
One Package Contains Ten or Twenty Vials or Ten Vials with Ten Sterile Needles with a 5 Micron Filter. Not all Pack Sizes may be Marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
LABORATOIRES THEA
12 rue Louis Blériot
63017 CLERMONT-FERRAND Cedex 2
France
Manufacturer:
BIOPHARMA S.R.L.
Via Delle Gerbere, 22/30 (loc. S. PALOMBA)
00134 ROMA (RM)
Italy
LABORATOIRES THEA
12 rue Louis Blériot
63017 CLERMONT-FERRAND Cedex 2
France
This Medicinal Product is Authorized in the Member States of the European Economic Area under the Following Names:
Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Italy, Iceland, Luxembourg, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Sweden, United Kingdom................................................................................................................................APROKAM
Cyprus, Greece, Spain..........................................................................................................PROKAM
Ireland………............................................................................................................................APROK
Date of Last Revision of the Package Leaflet: 07-08-2019
For more Detailed Information, Contact the Representative of the Marketing Authorization Holder:
Thea Polska Sp. z o.o.
ul. Cicha 7
00-353 Warsaw
www.thea.pl
Information Intended for Healthcare Professionals Only:
Incompatibilities
No Incompatibilities have been Reported with the Most Commonly Used Products during Cataract Surgery. Do not Mix this Medicinal Product with other Medicinal Products except those Listed below [Sodium Chloride 9 mg/ml (0.9%) Solution for Injection].
How to Prepare and Administer APROKAM
Single-Dose Vial for Injection into the Anterior Chamber of the Eye Only.
APROKAM must be Administered after Preparation of the Solution, by Injection into the Anterior Chamber of the Eye by the Eye Surgeon, in Recommended Aseptic Conditions during Cataract Surgery.
The Prepared Solution should be Visually Inspected and Used only if it is Colorless to Yellowish and Free from Visible Particles.
The Product should be Used Immediately after Preparation of the Solution and not Reused.
Recommended Dose of Cefuroxime is 1 mg in 0.1 ml of Sodium Chloride Injection Solution (9 mg/ml, 0.9%).
DO NOT INJECT A DOSE HIGHER THAN RECOMMENDED.
Vials are for Single Use Only
Each Vial is Intended for Use in a Single Patient Only. The Label of the Vial should be Affixed to the Patient's Documentation.
To Prepare APROKAM for Injection into the Anterior Chamber of the Eye, Follow these Instructions: 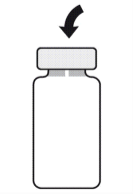 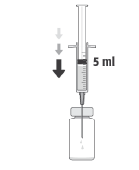 | |
Należy zdezynfekować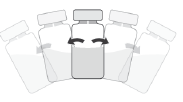 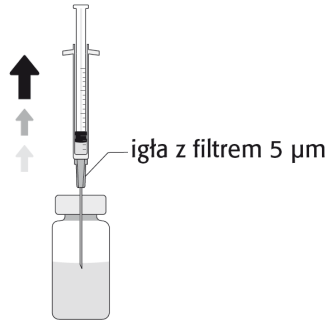 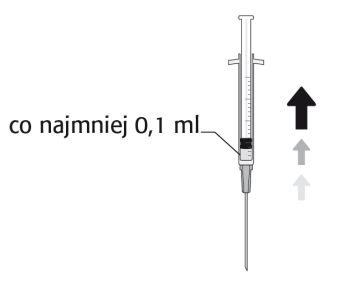 |
|
 |
|
After Use, Dispose of any Remaining Prepared Solution. Do not Store the Remaining Solution after Preparation for Reuse.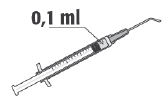 | |
Any Unused Medicinal Product or Waste Material should be Disposed of in Accordance with Local Requirements. Used Needles should be Placed in a Sharps Container for Medical Waste.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBiopharma S.r.L. Laboratoires Thea
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AprokamDosage form: Powder, 1.5 gActive substance: cefuroximeManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A. Zakłady Farmaceutyczne POLPHARMA S.A. Oddział Produkcyjny w DuchnicachPrescription requiredDosage form: Powder, 250 mgActive substance: cefuroximeManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A. Zakłady Farmaceutyczne POLPHARMA S.A. Oddział Produkcyjny w DuchnicachPrescription requiredDosage form: Powder, 500 mgActive substance: cefuroximeManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A. Zakłady Farmaceutyczne POLPHARMA S.A. Oddział Produkcyjny w DuchnicachPrescription required
Alternatives to Aprokam in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Aprokam in Spain
Alternative to Aprokam in Ukraine
Online doctors for Aprokam
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Aprokam – subject to medical assessment and local rules.











