

Aflegan

Ask a doctor about a prescription for Aflegan

How to use Aflegan
Package Leaflet: Information for the User
Aflegan, 7.5 mg/ml, Solution for Injection
Ambroxol Hydrochloride
Read the Package Leaflet Carefully Before Using the Medicinal Product, as it Contains Important Information for the Patient.
- Keep this Package Leaflet, You May Need to Read it Again.
- If You Have any Further Questions, Ask Your Doctor, Pharmacist, or Nurse.
- This Medicinal Product has been Prescribed to You. Do not Pass it on to Others. It may Harm them, even if their Symptoms are the Same as Yours.
- If You Experience any Side Effects, including those not Listed in this Package Leaflet, Tell Your Doctor, Pharmacist, or Nurse. See Section 4.
Package Leaflet Contents:
- 1. What Aflegan is and What it is Used for
- 2. Important Information Before Using Aflegan
- 3. How to Use Aflegan
- 4. Possible Side Effects
- 5. How to Store Aflegan
- 6. Package Contents and Other Information
1. What Aflegan is and What it is Used for
Aflegan Contains the Active Substance Ambroxol Hydrochloride - a Mucolytic Agent. This Medicinal Product Increases the Secretion of Mucus in the Airways and Improves its Transport, Making it Easier to Cough Up and Relieving Cough.
Aflegan is Used to Prevent Pulmonary Complications in Patients Receiving Intensive Medical Care after Surgery.
2. Important Information Before Using Aflegan
When Not to Use Aflegan:
- If the Patient is Allergic to Ambroxol Hydrochloride or any of the Other Ingredients of this Medicinal Product (Listed in Section 6).
Warnings and Precautions
Before Starting Treatment with Aflegan, Discuss it with Your Doctor, Pharmacist, or Nurse.
When Using Aflegan, be Cautious:
- If the Patient has Stomach or Duodenal Ulcer,
- If the Patient has Kidney Function Disorders and/or Severe Liver Disease,
- If the Patient's Cough Reflex is Weakened or has Respiratory Cilia Clearance Disorders (there is a Risk of Mucus Accumulation in the Airways),
- If the Patient has Bronchial Asthma (Aflegan may Initially Worsen Cough).
Severe Skin Reactions Associated with the Use of Ambroxol Hydrochloride have been Reported. If a Rash Appears (including Changes in Mucous Membranes, e.g., Mouth, Throat, Nose, Eyes, Genital Organs), Discontinue Use of Aflegan and Contact Your Doctor Immediately.
Note:
After Administration of the Medicinal Product, Cough should be Induced to Facilitate Expectoration of the Thinned Mucus (or the Mucus should be Aspirated).
The Medicinal Product should not be Used Directly Before Sleep.
Aflegan and Other Medicinal Products
Tell Your Doctor about all Medicinal Products You are Currently Using or have Recently Used, as well as those You Plan to Use.
- Ambroxol Increases the Concentration of Antibiotics (amoxicillin, cefuroxime, erythromycin, doxycycline) in Lung Tissue.
- Cough Suppressants (e.g., Codeine) Inhibit the Cough Reflex and Make it Difficult to Cough Up Thinned Mucus, so Ambroxol should not be Used at the Same Time as Cough Suppressants.
- No Clinically Significant Adverse Interactions (Interactions) between Ambroxol and Other Medicinal Products have been Found.
Using Aflegan with Food and Drink
Aflegan can be Used Independently of Meals.
Pregnancy, Breast-feeding, and Fertility
If You are Pregnant or Breast-feeding, Think You may be Pregnant or are Planning to have a Child, Consult Your Doctor or Pharmacist Before Using this Medicinal Product.
Pregnancy
Use of Aflegan is not Recommended During Pregnancy, Especially in the First Trimester.
Breast-feeding
Ambroxol Passes into Breast Milk. Use of Aflegan is not Recommended During Breast-feeding.
Driving and Using Machines
There is no Evidence of the Medicinal Product's Influence on the Ability to Drive and Use Machines.
No Studies have been Conducted on the Influence of Aflegan on the Ability to Drive and Use Machines.
Aflegan Contains Sodium
The Medicinal Product Contains Less than 1 mmol (23 mg) of Sodium per Ampoule, i.e., the Medicinal Product is Considered "Sodium-free".
3. How to Use Aflegan
This Medicinal Product should Always be Used in Accordance with the Doctor's Recommendations. In Case of Doubts, Consult Your Doctor.
Recommended Dose:
Adults:15 mg (1 Ampoule) 2 to 3 Times a Day, and in Severe Cases 30 mg (2 Ampoules) 2 to 3 Times a Day.
Patients with Kidney Function Disorders:
In Patients with Renal Insufficiency, the Doctor may Reduce the Dose or Prolong the Intervals between Doses.
Method of Administration:
Aflegan is Administered by Slow Intravenous Injection.
The Medicinal Product can also be Administered by Drip Infusion, after Prior Dilution in a Glucose Solution, Fruktose, 0.9% Sodium Chloride Solution, or Ringer's Solution.
Aflegan should not be Mixed with Solutions with a pH Higher than 6.2, as this may Cause the Solution to Become Turbid and Precipitate - Ambroxol Base.
Using a Higher Dose of Aflegan than Recommended
No Specific Symptoms of Ambroxol Overdose have been Described in Humans.
After Using a Higher Dose of Aflegan than Recommended, the Side Effects Listed in Section 4 "Possible Side Effects" may Occur. In this Case, Contact Your Doctor Immediately. Symptomatic Treatment is Recommended.
Missing a Dose of Aflegan
If a Dose of the Medicinal Product is Missed, it should be Taken as Soon as Possible. However, if the Time for the Next Dose is Approaching, the Missed Dose should be Omitted. Do not Take a Double Dose to Make up for the Missed Dose.
Stopping Treatment with Aflegan
Stopping Treatment with Aflegan does not Cause any Adverse Effects, Except for the Possible Worsening of Mucus Stagnation in the Airways.
In Case of any Further Doubts Regarding the Use of this Medicinal Product, Consult Your Doctor or Pharmacist.
4. Possible Side Effects
Like all Medicinal Products, Aflegan can Cause Side Effects, although not Everybody gets them.
If You Experience any of the Following Side Effects, Stop Using the Medicinal Product and Contact Your Doctor Immediately:
- Anaphylactic Reactions, including Anaphylactic Shock, Angioedema (Rapidly Progressing Swelling of the Skin, Subcutaneous Tissue, Mucous Membranes, or Submucosal Tissue) and Pruritus
- Severe Skin Reactions (including Erythema Multiforme, Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis, and Acute Generalized Exanthematous Pustulosis)
The Frequency of the Above-mentioned Side Effects is Unknown (Cannot be Determined from Available Data).
Additionally, the Following Side Effects may Occur:
- Rarely (may Occur in up to 1 in 1,000 People):
- Hypersensitivity Reactions
- Rash, Urticaria
- Heartburn, Constipation
Very Rarely (may Occur in up to 1 in 10,000 People):
- Facial Edema, Dyspnea, Increased Body Temperature, Chills
Frequency Unknown (Frequency cannot be Determined from Available Data):
- Nausea, Vomiting, Diarrhea, Dyspepsia, and Abdominal Pain.
Reporting Side Effects
If You Experience any Side Effects, including those not Listed in this Package Leaflet, Tell Your Doctor, Pharmacist, or Nurse. Side Effects can be Reported Directly to the Department for Monitoring of Adverse Reactions to Medicinal Products, the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side Effects can also be Reported to the Marketing Authorization Holder.
Reporting Side Effects will Help to Gather more Information on the Safety of the Medicinal Product.
5. How to Store Aflegan
Store Below 25°C.
Keep the Medicinal Product out of the Sight and Reach of Children.
Do not Use this Medicinal Product after the Expiry Date Stated on the Packaging. The Expiry Date refers to the Last Day of the Month.
Medicinal Products should not be Disposed of via Wastewater or Household Waste. Ask Your Pharmacist how to Dispose of Medicinal Products that are no Longer Needed. This will Help Protect the Environment.
6. Package Contents and Other Information
What Aflegan Contains
- The Active Substance of the Medicinal Product is Ambroxol Hydrochloride. 1 ml of the Solution for Injection Contains 7.5 mg of Ambroxol Hydrochloride. 1 Ampoule (2 ml Solution) Contains 15 mg of Ambroxol Hydrochloride.
- The Other Ingredients are: Sodium Chloride, Disodium Phosphate Dodecahydrate, Citric Acid Monohydrate, Water for Injections.
What Aflegan Looks like and What the Package Contains
Aflegan is a Clear, Colorless to Light Yellow Solution.
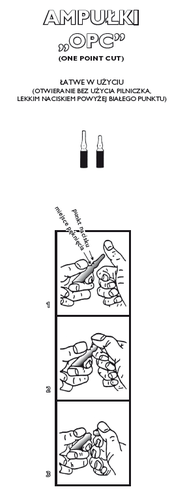
The Carton Contains 10 Ampoules of 2 ml Solution for Injection Each. The Orange OPC Ampoules have a White Ceramic Point and a Red Identification Ring.
Marketing Authorization Holder:
Bausch Health Ireland Limited
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Manufacturer:
Przedsiębiorstwo Farmaceutyczne Jelfa SA
ul. Wincentego Pola 21
58-500 Jelenia Góra
Date of Last Revision of the Package Leaflet: -----------------------------------------------------------------------------------------------------------------
Information Intended for Healthcare Professionals Only:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterPrzedsiębiorstwo Farmaceutyczne Jelfa S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AfleganDosage form: Syrup, 15 mg/5 mlActive substance: ambroxolManufacturer: Sopharma PLCPrescription not requiredDosage form: Syrup, 30 mg/5 mlActive substance: ambroxolPrescription not requiredDosage form: Syrup, 15 mg/5 mlActive substance: ambroxolPrescription not required
Alternatives to Aflegan in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Aflegan in Spain
Alternative to Aflegan in Ukraine
Online doctors for Aflegan
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Aflegan – subject to medical assessment and local rules.














