
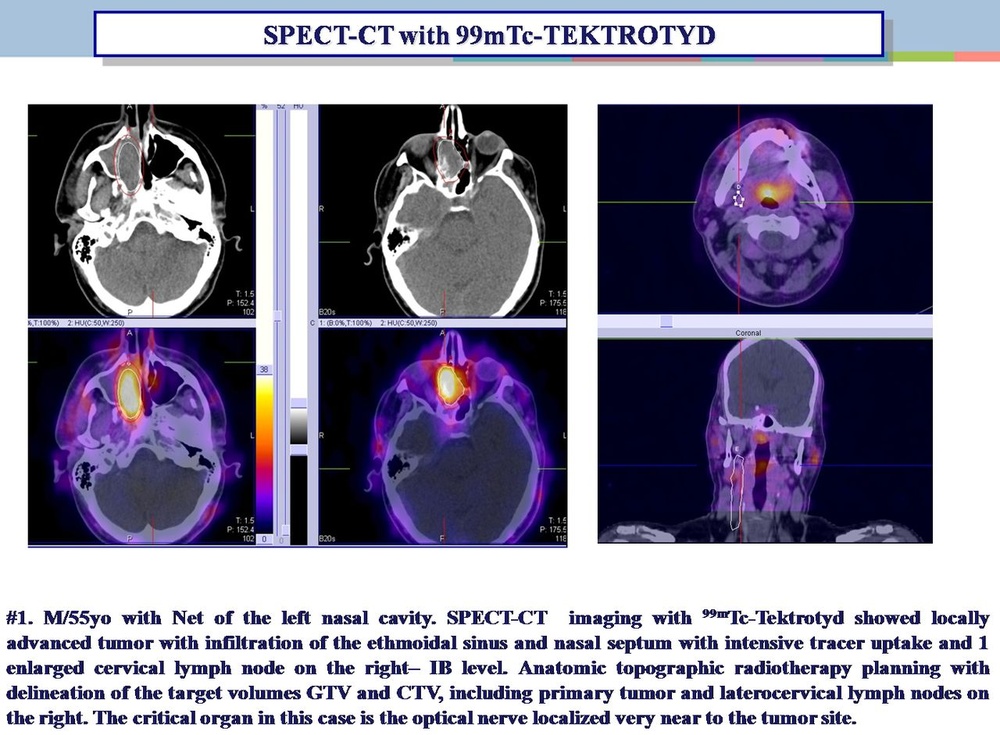
99mtc - Tektrotid

Ask a doctor about a prescription for 99mtc - Tektrotid

How to use 99mtc - Tektrotid
Package Leaflet: Information for the User
Tc-Tektrotyd, 16 micrograms, kit for preparation of a radiopharmaceutical
Radiopharmaceutical
Hynic-[D-Phe, Tyr-Oktreotyd]·TFA
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or a specialist in nuclear medicine who is supervising the examination.
- If the patient experiences any adverse reactions, including any adverse reactions not listed in this leaflet, they should inform the doctor or specialist in nuclear medicine.
Table of Contents of the Leaflet:
- 1. What Tc-Tektrotyd is and what it is used for
- 2. Important information before using Tc-Tektrotyd
- 3. How to use Tc-Tektrotyd
- 4. Possible adverse reactions.
- 5. How to store Tc-Tektrotyd
- 6. Contents of the package and other information.
1. What Tc-Tektrotyd is and what it is used for
Tc-Tektrotyd, a kit for preparation of a radiopharmaceutical, is intended exclusively for diagnostic examinations. After intravenous administration, it accumulates in areas with somatostatin receptors. These receptors are present in healthy individuals, but their excess is found in various diseases. Detecting the site of excessive somatostatin receptor accumulation can help the doctor choose the most effective treatment method.
Tc-Tektrotyd is radioactive, so its accumulation in organs is recorded by special detection devices, which allow for the creation of images showing the distribution of the radiopharmaceutical in the body. These images accurately show the areas of radiopharmaceutical accumulation. This provides the treating doctor with important information, such as the presence and location of pathological changes in the body. Scintigraphy, or radioisotope examination, is a recognized diagnostic method used to assess the functional state of various body organs in many different diseases.
2. Important information before using Tc-Tektrotyd
When not to use Tc-Tektrotyd
- if the patient has been found to be hypersensitive (allergic) to Hynic-[D-Phe, Tyr-Oktreotyd]·TFA or to any of the other components of Tc-Tektrotyd (practically no allergic reactions have been observed after administration of the preparation);
- if the patient is pregnant;
- if the patient is breastfeeding, then breastfeeding should be discontinued for at least 72 hours after administration of the radiopharmaceutical, due to the potential risk to the child's health. Milk should be expressed, but not given to the child.
Warnings and precautions
Particular caution should be exercised when using Tc-Tektrotyd:
- if the patient has been diagnosed with kidney failure;
- if the patient has been diagnosed with liver disease.
If any of the above information applies to the patient, they should immediately inform their doctor.
Children and adolescents
The use of Tc-Tektrotyd is not recommended in patients under 18 years of age, as no clinical trials have been conducted in this age group.
The use of Tc-Tektrotyd involves the administration of a small dose of radiation, but the doctor ordering the examination will weigh the benefits and risks associated with its use.
To reduce the dose of radioactivity absorbed by the urinary bladder walls, the patient should drink plenty of fluids and urinate frequently in the hours following the examination.
Tc-Tektrotyd and other medicines
Patients treated with somatostatin analogs (both "cold" and radiolabeled with radioactive isotopes) should discontinue these medications:
- short-acting analogs - at least 3 days before the planned examination;
- long-acting analogs:
- lanreotide - at least 3 weeks before the planned examination;
- octreotide - at least 5 weeks before the planned examination.
If any of the above information applies to the patient, they should immediately inform their doctor.
There is limited data on potential interactions with other medicines. Before the planned examination, the patient should inform their doctor about all medicines currently being used or recently used, as well as any medicines planned for use.
Tc-Tektrotyd with food and drink
To obtain the best possible quality of the scintigraphic image, the patient requires proper preparation before administration of the radiopharmaceutical. Unless the doctor recommends otherwise, a light diet is recommended on the day before the examination. The doctor may recommend the administration of laxatives on the day before the examination. On the day of the examination, the patient should fast until the completion of the first image registration.
The treating doctor may recommend a different preparation method for the patient. In case of doubts, this should be clarified with the treating doctor.
Pregnancy and breastfeeding
Pregnancy is an absolute contraindication.
Before using any medicine, consult a doctor or pharmacist.
Driving and operating machinery
No studies have been conducted on the effect of the product on the ability to drive vehicles or operate machinery.
However, it is not expected that Tc-Tektrotyd will affect the ability to drive or operate machinery.
3. How to use Tc-Tektrotyd
Dosage and administration
Tc-Tektrotyd should be used in adults over 18 years of age.
The recommended dose for a single examination in an adult is between 370 and 925 MBq.
Tc-Tektrotyd, labeled with radioactive sodium technetate(VII) [Tc], is administered in a single intravenous injection before the scintigraphic examination.
The scintigraphic examination should be started 2 to 4 hours after administration of Tc-Tektrotyd. Depending on the doctor's decision, the examination can also be started 10 minutes, 1 hour, or 24 hours after administration of Tc-Tektrotyd.
The remaining radioactive Tc-Tektrotyd molecules in the body will lose their radioactive properties within 2-3 days after administration.
The storage, use, and disposal of radiopharmaceuticals are regulated by special regulations. Therefore, the use of Tc-Tektrotyd is only possible in specialized facilities by personnel trained in this field.
Use of a higher dose of Tc-Tektrotyd than recommended
In the event of an overdose of Tc-Tektrotyd, the doctor may recommend that the patient drink plenty of fluids and urinate frequently to eliminate the excess radioactive preparation.
4. Possible adverse reactions
Like all medicines, Tc-Tektrotyd can cause adverse reactions, although not everybody gets them. Very rarely, a transient headache or abdominal pain may occur immediately after administration.
Reporting adverse reactions
If any adverse reactions occur, including any adverse reactions not listed in the leaflet, the patient should inform their doctor or specialist in nuclear medicine. Adverse reactions can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products {current address, phone number, and fax of the Department}
e-mail: [email protected].
Reporting adverse reactions can help gather more information on the safety of the medicine.
5. How to store Tc-Tektrotyd
Store in a place inaccessible and invisible to children.
Store in a refrigerator (2°C-8°C).
During transport, a temperature of up to 35°C is allowed for no more than 7 days.
After labeling, the prepared preparation should be stored for no more than 6 hours, at a temperature below 25°C, in a shield absorbing ionizing radiation.
The storage of radiopharmaceuticals should be in accordance with applicable regulations.
The storage conditions and expiration date are stated on the packaging. Do not use Tc-Tektrotyd after the expiration date stated on the label.
Trained personnel at a specialized facility will ensure proper storage conditions for the Tc-Tektrotyd preparation kit.
6. Contents of the package and other information
What Tc-Tektrotyd contains
Vial I: Hynic-[D-Phe, Tyr-Oktreotyd]·TFA, tricine (N-[tris(hydroxymethyl)methyl]glycine), tin(II) chloride dihydrate, mannitol, nitrogen.
Vial II: EDDA (ethylenediamine-N,N'-diacetic acid), disodium hydrogen phosphate dodecahydrate, sodium hydroxide, nitrogen.
The active substance of the medicine is HYNIC-[D-Phe, Tyr-Oktreotyd]·TFA
What Tc-Tektrotyd looks like and what the package contains
The package contains a kit of two 10 ml glass vials (Vial I and Vial II). Each vial is closed with a rubber stopper and an aluminum cap. The vials are packed in cardboard boxes. Vials I and II contain the components for preparing the Tc-Tektrotyd radiopharmaceutical.
Marketing authorization holder and manufacturer
National Centre for Nuclear Research
ul. Andrzeja Sołtana 7
05-400 Otwock
Phone: 22 7180700
Fax: 22 7180350
e-mail: [email protected]
Date of the last update of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterNarodowe Centrum Badań Jądrowych
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to 99mtc - TektrotidDosage form: Kit, 20 mcgActive substance: technetium (99mTc) hynic-octreotideManufacturer: Narodowe Centrum Badań JądrowychPrescription not requiredDosage form: Solution, 1 GBq/1ml on the day and time of referenceActive substance: fluorocholine (18F)Prescription requiredDosage form: Solution, 3000 MBq/ml at the time of calibrationActive substance: fludeoxyglucose (18F)Prescription not required
Alternatives to 99mtc - Tektrotid in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to 99mtc - Tektrotid in Ukraina
Alternative to 99mtc - Tektrotid in Hiszpania
Online doctors for 99mtc - Tektrotid
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for 99mtc - Tektrotid – subject to medical assessment and local rules.











